DIA MS Host Cell Protein Profiling Solutions
Host cell proteins (HCPs) are unintended protein contaminants derived from host expression systems such as CHO, E. coli, and HEK293, which may remain as process-related impurities in biopharmaceutical production. Even at low concentrations, residual host cell proteins can affect drug safety, stability, and immunogenicity, making their accurate detection and quantification critical for biologics manufacturing, quality control, and regulatory compliance. The ability to comprehensively profile host cell proteins is essential for ensuring product purity and meeting stringent IND and BLA regulatory requirements.
Traditional enzyme-linked immunosorbent assay (ELISA) has been the standard method for host cell protein detection, but its limited antibody coverage and lack of specificity for low-abundance or novel contaminants pose significant challenges. To overcome these limitations, mass spectrometry (MS)-based approaches provide greater sensitivity, specificity, and comprehensive proteome coverage, making them an indispensable tool for HCP characterization.
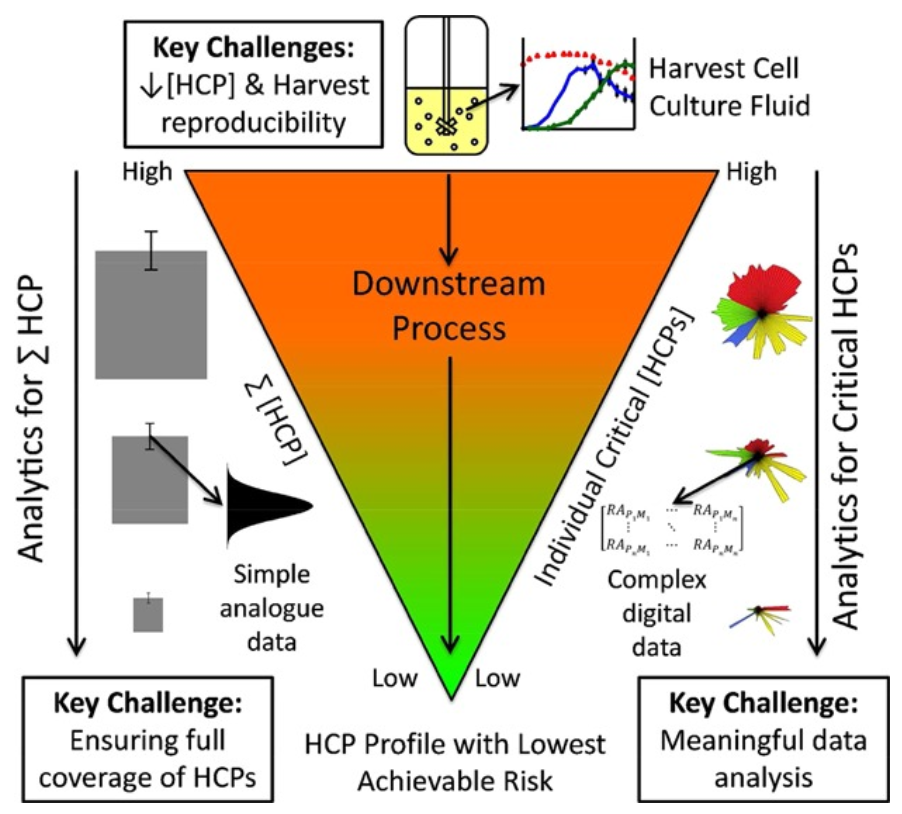
Figure 1. Overview of the HCP Landscape in the Context of Biologics Manufacture
Among MS-based techniques, Data-Independent Acquisition mass spectrometry (DIA-MS) has proven to be a high-throughput, unbiased, and quantitative solution for host cell protein profiling. Unlike Data-Dependent Acquisition (DDA-MS), which selectively analyzes precursor ions and can miss low-abundance proteins, DIA-MS captures all detectable peptides within a broad dynamic range, ensuring a more comprehensive and reproducible quantification of host cell proteins in complex biological samples. This approach enables the identification of both high- and low-abundance impurities, providing a deeper understanding of HCP composition, clearance efficiency, and potential risks associated with residual proteins in biopharmaceutical production. By integrating DIA-MS into HCP analysis, researchers can achieve a more robust and regulatory-compliant assessment of process-related impurities, ultimately enhancing the safety, efficacy, and consistency of biologic therapeutics.
Service at MtoZ Biolabs
To support biopharmaceutical development and regulatory compliance, MtoZ Biolabs offers DIA MS Host Cell Protein Profiling Solutions, providing high-throughput, quantitative, and highly sensitive HCP analysis. Our DIA-MS platform enables comprehensive impurity profiling, ensuring accurate HCP quantification, process optimization, and enhanced drug safety assessment. We also provide conventional HCP profiling tailored to client needs. Simply send your samples and specify your requirements, and we will handle protein extraction, mass spectrometry analysis, data processing, and bioinformatics analysis.
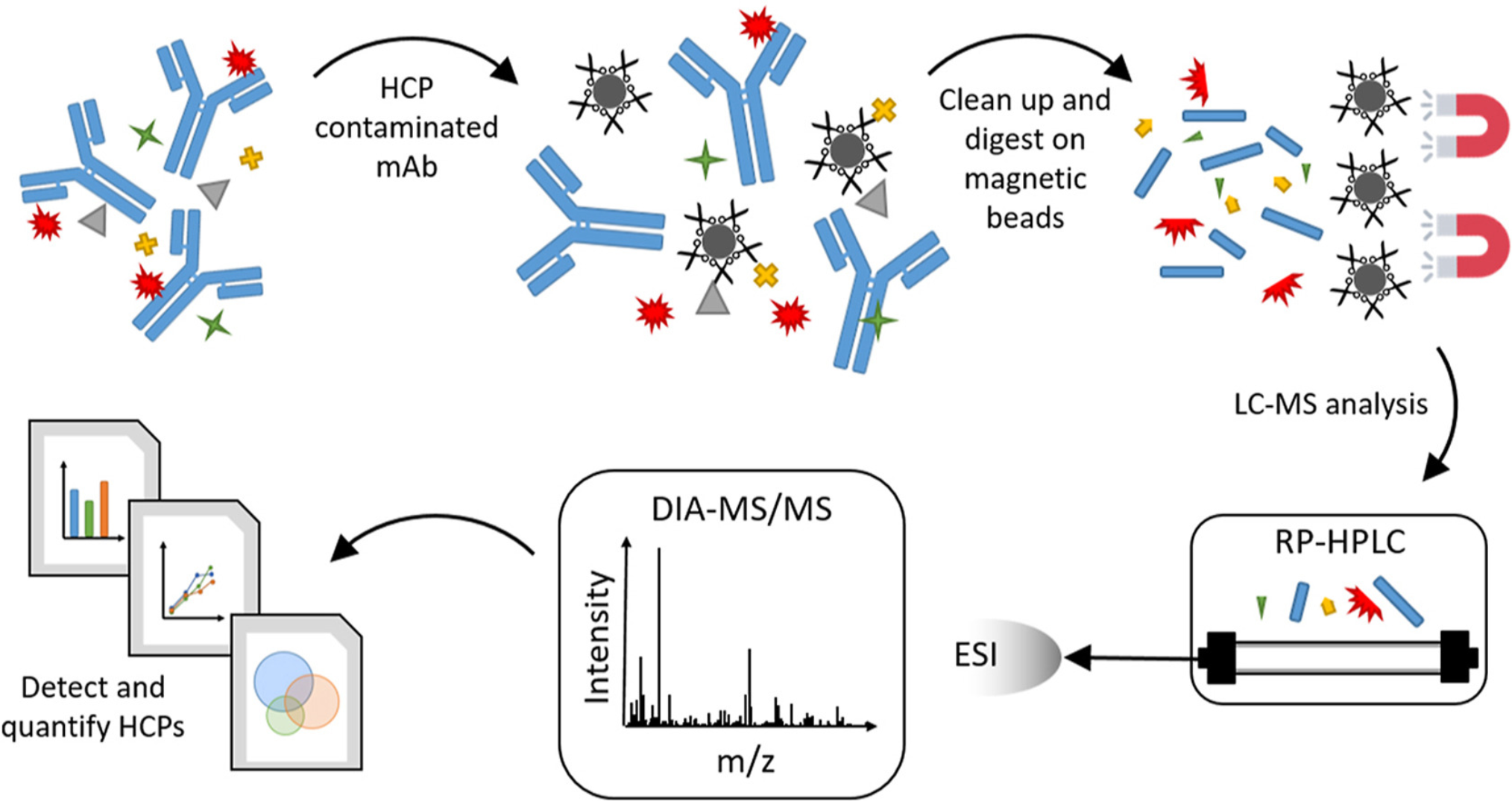
Figure 2. Detection and Quantitation of Host Cell Proteins in Monoclonal Antibody Drug Products
Service Advantages
1. High Sensitivity
DIA MS Host Cell Protein Profiling Solutions achieve unbiased, high-throughput detection of both high- and low-abundance HCPs, ensuring comprehensive impurity profiling.
2. Reproducible Analysis
DIA MS Host Cell Protein Profiling Solutions enable precise, label-free quantification, providing high reproducibility across samples.
3. Comprehensive HCP Characterization
Detects a broad range of HCPs beyond the limitations of ELISA, including proteins lacking antibody coverage or present at trace levels.
4. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
MtoZ Biolabs’ DIA MS Host Cell Protein Profiling Solutions support every stage of biopharmaceutical development.
1. Bioprocess Development: Identify and quantify HCP impurities in early-stage biologic production to guide process design and optimization.
2. Impurity Removal and Clearance Validation: Monitor HCP removal efficiency at purification steps to ensure effective impurity reduction and minimize immunogenic risk.
3. Quality Control and Batch Consistency: Assess HCP profiles across production batches to maintain product quality and lot-to-lot consistency.
4. Regulatory Submission: Provide accurate HCP data to support biopharmaceutical companies in meeting regulatory submission requirements.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
MtoZ Biolabs provides accurate, reproducible, and regulatory-compliant DIA MS Host Cell Protein Profiling Solutions for biopharmaceutical development. Contact us to explore tailored solutions for your research.
How to order?







