Dendritic Cell-derived Exosomes-based Vaccine
Dendritic Cell-derived Exosomes-based Vaccine development service enables the processing of tumor-specific antigens within dendritic cells (DCs) to induce the secretion of functional dendritic cell-derived exosomes (DEXs), which are used to activate T cell immune responses and achieve targeted tumor immunotherapy. This service is applicable to vaccine development and mechanistic studies across various tumor types.
As a next-generation immunotherapy platform, exosome-based vaccines are rapidly transforming the landscape of cancer vaccine development. Among them, DEXs are considered ideal substitutes for conventional cell-based vaccines due to their innate antigen-presenting capability and immunogenic activity.
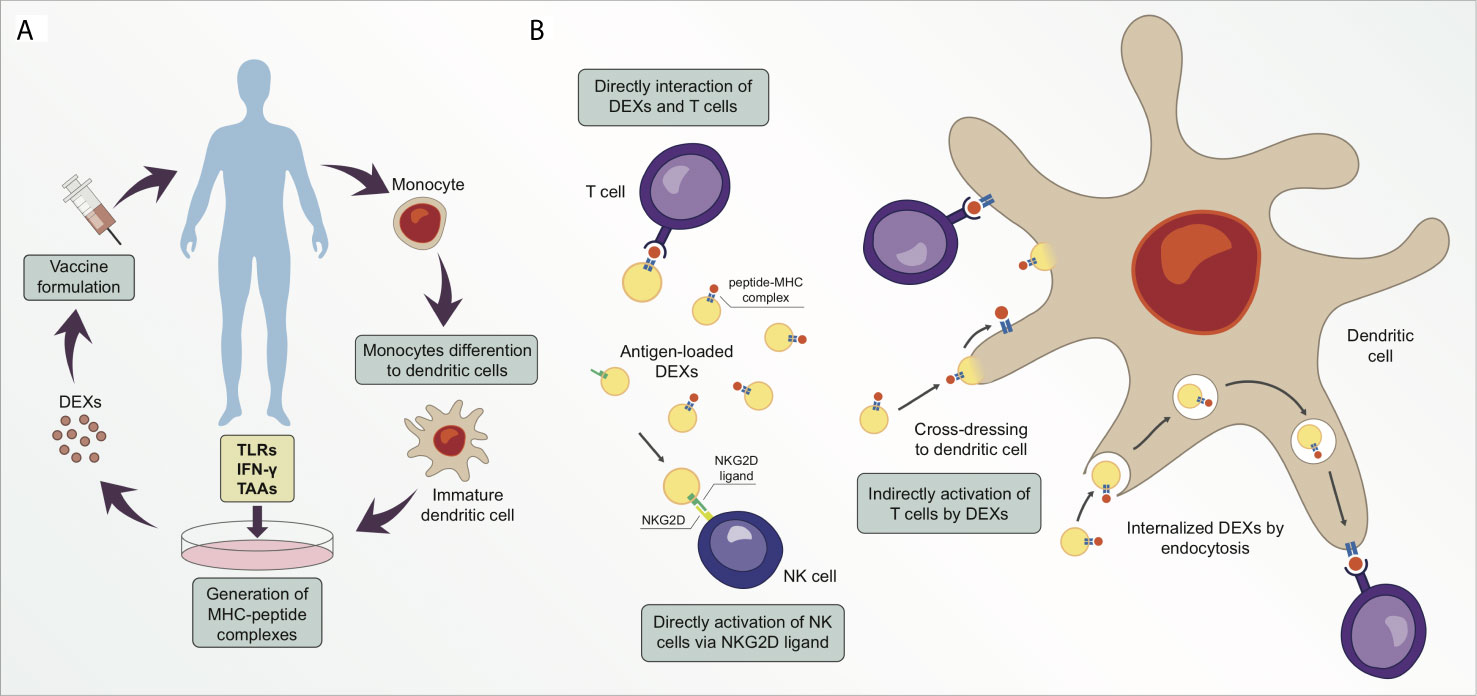
Santos P. Almeida F. Frontiers in Immunology. 2021.
DCs are key antigen-presenting cells that bridge innate and adaptive immunity. They play a pivotal role in priming naive T cells and initiating specific immune responses. Exosomes (DEXs) secreted by DCs carry MHC class I and class II molecules, co-stimulatory molecules, and specific antigens, which can mimic the functions of DCs, activate T cells, and induce immune responses. Studies have shown that DEXs outperform conventional DC vaccines in overcoming tumor-induced immunosuppression and exhibit enhanced bioavailability and biological stability.
Leveraging advanced exosome engineering and immune cell platforms, MtoZ Biolabs provides Dendritic Cell-derived Exosomes-based Vaccine development service to harness DEXs as novel vaccine carriers for immunotherapeutic strategies targeting a wide range of diseases, including cancer and chronic infections. Our comprehensive service encompasses the entire workflow—from exosome isolation and characterization to vaccine design and functional validation—helping clients to advance exosome-based vaccine research and applications.
Analysis Workflow
MtoZ Biolabs offers a comprehensive, customized one-stop service for the development of dendritic cell-derived exosomes-based vaccines. The workflow includes:
1. DCs Culture and Antigen Loading
Monocytes are isolated from peripheral blood mononuclear cells (PBMCs) and induced to differentiate into DCs. These DCs are then incubated with specific tumor antigens or tumor lysates to load the target antigen.
2. DEXs Isolation and Purification
DEXs are isolated and purified from culture supernatants using ultracentrifugation, density gradient centrifugation, or size-exclusion chromatography (SEC).
3. DEXs Characterization
Comprehensive characterization of DEXs is performed using nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and Western blotting to assess particle size, morphology, surface markers, and antigen-presenting capabilities.
4. Functional Validation
The immunogenicity of DEXs is evaluated both in vitro and in vivo, including T cell proliferation, cytokine release, and anti-tumor activity assays.
Applications
Cancer Immunotherapy
Dendritic Cell-derived Exosomes-based Vaccines development service can be used to study and construct personalized vaccines targeting specific tumor antigens.
Vaccine Mechanism Studies
Facilitates understanding of DEX-mediated activation of CD4⁺/CD8⁺ T cells, tumor immunity pathways, and memory cell formation.
Immunoadjuvant Platform Development
DEXs can function as natural adjuvants to enhance the efficacy of other vaccine platforms.
Alternative to DC Cell Vaccines
Provides a scalable and standardized solution for cases where DC function is impaired or hard to implement clinically.
Service Advantages
1. Tailored Solutions: End-to-end customization from DCs culture, DEXs purification to functional validation, ensuring targeted and efficient research outcomes.
2. High-Quality Results: With extensive experience and advanced technical platforms, we deliver DEXs with high purity, potency, and reproducibility to meet rigorous experimental standards.
3. Expert Support: Our experienced team offers full technical consultation and support throughout your project, ensuring smooth progress and problem resolution.
4. One-Time Charge: Transparent pricing with no hidden fees or additional costs.
FAQ
Q. Do DEXs retain sufficient antigen-presenting and co-stimulatory capacity?
Yes. High-quality DEXs exhibit stable membrane expression of MHC-I/MHC-II–peptide complexes and co-stimulatory molecules such as CD80, CD86, and ICAM-1, enabling full T cell activation. We verify expression via Western blot and flow cytometry, and further confirm function through T cell co-culture assays.
Q. Can I load personalized tumor antigens? What formats are supported?
Absolutely. We support various antigen formats, including peptides, full-length proteins, mRNA, and tumor lysates. Personalized antigen presentation strategies are designed based on your research needs. Antigen processing is validated by Western blot or functional assays to ensure DEX integrity and immunological activity.
Case Study
In this study, mouse bone marrow-derived dendritic cells were cultured in vitro and loaded with hepatocellular carcinoma (HCC) antigens during their maturation phase. The secreted exosomes (DEXs) were collected to create a DEX-based vaccine. This vaccine was combined with microwave ablation (MWA) therapy in a murine HCC model. The combination significantly enhanced systemic anti-tumor immunity, including increased CD8⁺ T cell infiltration, reduced Treg cell ratios, elevated IFN-γ levels, and suppressed IL-10 expression. Notably, the treatment also inhibited growth of distant untreated tumors, supporting the potential of DEX vaccines as immunotherapeutic enhancers alongside local ablation strategies.
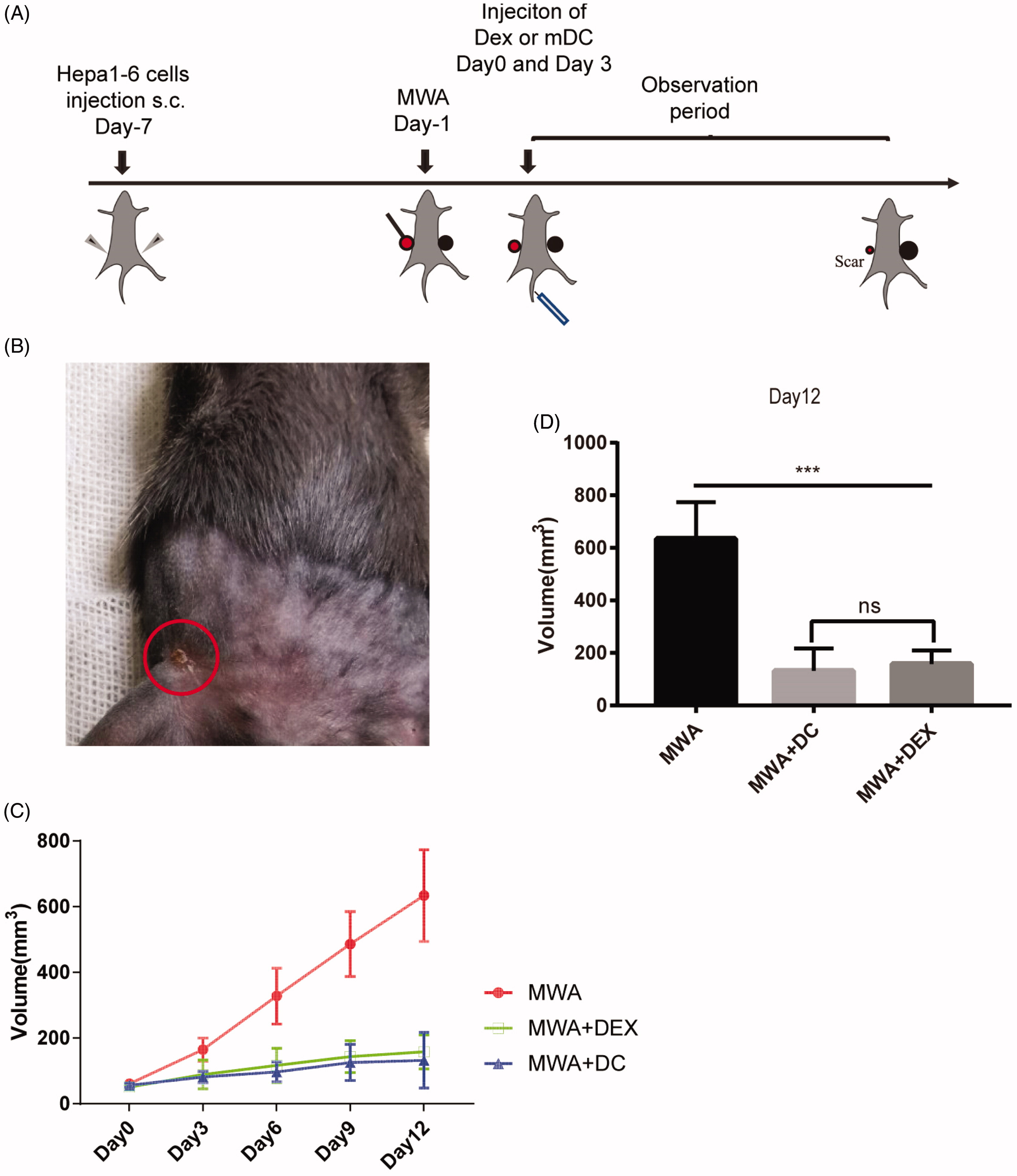
Zhong X. et al. International Journal of Hyperthermia. 2020.
How to order?







