Comparison and Advantages of Native Mass Spectrometry over Other MS Techniques
Mass spectrometry has long been a fundamental analytical tool in modern life sciences and biomedical research, enabling the structural and functional characterization of biomolecules such as proteins and nucleic acids. Based on the preservation or disruption of biomolecular native states, mass spectrometry can be broadly classified into native mass spectrometry and denatured mass spectrometry. What distinguishes these two approaches? In what research contexts are they most suitable? This review provides a comprehensive comparison.
Core Principle Comparison
1. Native Mass Spectrometry
(1) Principle: Analyzes intact proteins or protein complexes under near-physiological conditions, preserving their native conformation, post-translational modifications (PTMs), and non-covalent interactions.
(2) Key Technologies
①Soft ionization techniques (e.g., electrospray ionization, ESI)
②High-resolution mass analyzers (Orbitrap, Q-TOF)
③Low-energy dissociation methods (soft CID, SID)
2. Denatured Mass Spectrometry
(1) Principle: Proteins are denatured using chemical agents (e.g., urea, SDS), followed by enzymatic digestion into peptides before mass spectrometric analysis. This approach is particularly suited for high-throughput protein identification and post-translational modification site mapping.
(2) Key Technologies
①High-energy fragmentation techniques (HCD, CID)
②Liquid chromatography coupling (LC-MS/MS)
Technical Features and Comparative Advantages of Different MS Techniques
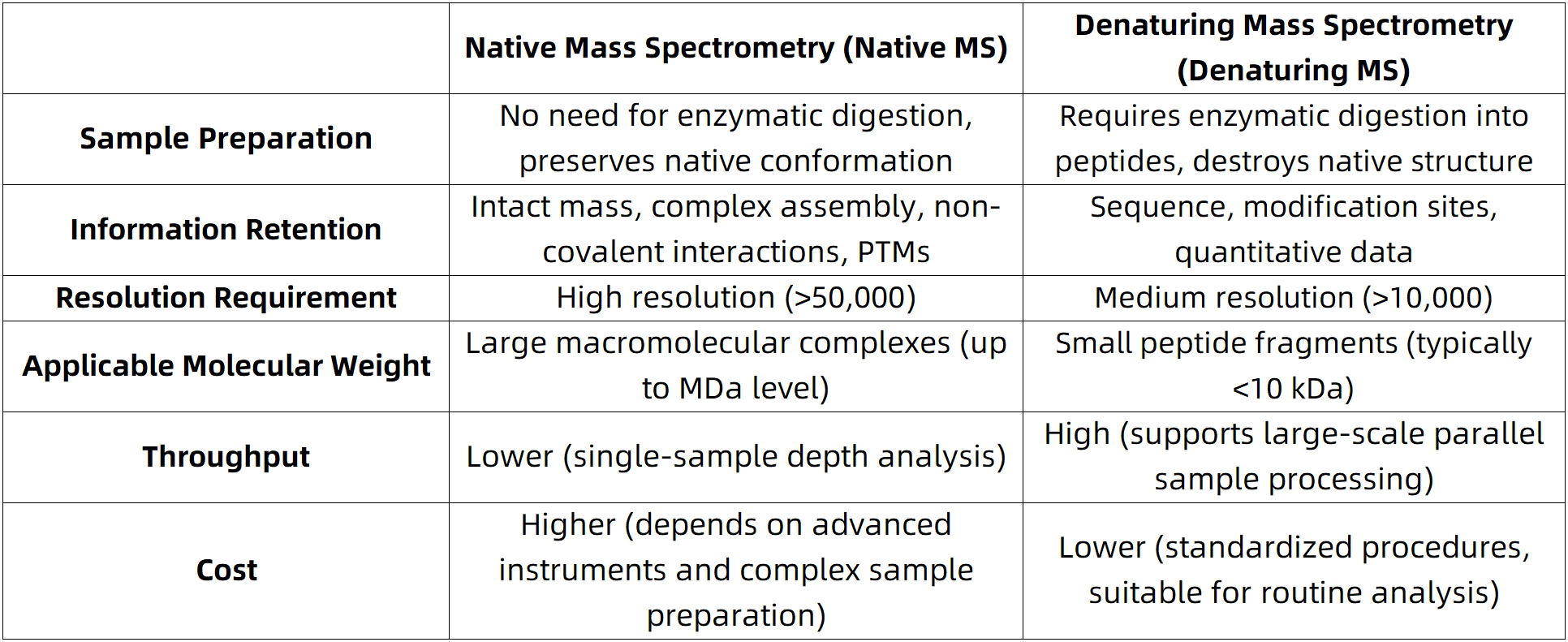
Figure 1
Typical Applications
1. Native Mass Spectrometry
(1) Structural Biology: Resolving native conformations of protein complexes, such as antibody-antigen interactions and viral capsid assemblies.
(2) Drug Development: Evaluating the glycosylation patterns, aggregation states, and stability of monoclonal antibodies.
(3) Dynamic Interaction Studies: Characterizing protein-small molecule interaction kinetics and associated conformational changes, such as those influenced by kinase inhibitors.
(4) Post-Translational Modification Analysis: Direct detection of intact protein modifications, including phosphorylation, acetylation, and other regulatory modifications.
2. Denatured Mass Spectrometry
(1) High-Throughput Proteomics: Identifying thousands of proteins from complex biological samples, such as serum and tissue extracts, with high sensitivity and speed.
(2) High-Resolution Mapping of Post-Translational Modifications: Determining the precise modification sites of phosphorylation, ubiquitination, and other regulatory marks, with applications in biomarker discovery (e.g., cancer diagnostics).
(3) Quantitative Proteomics: Comparing protein abundance differences through isotope labeling (iTRAQ, TMT) or label-free (LFQ) quantification methods.
Choosing Between Native and Denatured Mass Spectrometry
The selection of MS techniques depends on the specific research objectives:
1. If the primary goal is to verify protein expression fidelity, molecular weight consistency, and post-translational modifications, denatured mass spectrometry is the preferred approach.
2. If the research focuses on understanding the native state of protein complexes, their binding interactions, and structural stability, native mass spectrometry is more suitable.
As the fields of biomedicine and structural biology continue to advance rapidly, native mass spectrometry and denatured mass spectrometry have become indispensable, complementary analytical tools. A strategic combination of both approaches allows researchers to achieve a more comprehensive understanding of biomolecular structures and functions, providing robust scientific insights for drug development and disease mechanism studies. For expert guidance on experimental design and mass spectrometry analysis services, feel free to contact us—we are here to support your research with high-efficiency solutions!
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







