Chemical Proteomics: Principles, Methods, and Applications
-
Diverse Chemical Probe Platforms: Support both commercially available probes and custom synthesis, including bioorthogonal modifications
-
High-sensitivity Mass Spectrometry Systems: Featuring Orbitrap Exploris 480 and TripleTOF platforms to ensure exceptional detection sensitivity
-
Target Discovery and Pathway Analysis: Coupled with high-throughput bioinformatics pipelines for identification of enriched targets and their biological roles
In modern proteomics research, data-dependent and targeted acquisition strategies such as DDA, DIA, and PRM have been widely adopted to reveal protein expression profiles and abundance dynamics. However, these approaches primarily emphasize the presence and quantity of proteins, making them insufficient for directly characterizing functional states, such as enzymatic activity, drug-binding sites, or conformational changes. To address this limitation, the field of Chemical Proteomics has emerged. By incorporating reactive probes or clickable functional groups and leveraging mass spectrometry, this approach enables selective labeling and precise analysis of active proteins, functional sites, and potential drug targets. As a result, it has become an essential tool in drug discovery and functional protein analysis.
Basic Principles of Chemical Proteomics
1. What Is Chemical Proteomics?
Chemical proteomics is a research strategy that integrates small-molecule chemical probes with proteomic techniques. It employs covalent chemistry, specific affinity interactions, or bioorthogonal reactions to label, enrich, and detect target proteins. This enables the identification of protein activity states, modification sites, and drug-binding targets.
2. Three Key Elements
(1) Functional chemical probes: Molecules designed to specifically recognize certain classes of proteins or catalytic sites, such as serine esterases or cysteine proteases.
(2) Selective modification reactions: Chemical reactions such as nucleophilic substitution or click chemistry that enable site-specific labeling.
(3) High-resolution mass spectrometry: Utilized for qualitative and quantitative analysis of labeled proteins or peptides.
Mainstream Strategies and Method Categories
1. Activity-Based Protein Profiling (ABPP)
This strategy employs covalent probes to selectively label active enzyme sites and is widely used for profiling functional enzyme classes and screening inhibitors. Probes are typically composed of a recognition element, a reactive group, and an enrichment handle. ABPP has been applied in profiling enzymatic activity for classes such as esterases and proteases and in the development of novel inhibitors.
2. Target Profiling of Covalent Inhibitors
In this approach, small-molecule inhibitors are rationally designed to incorporate chemical probe functionalities. By combining competitive binding assays with click chemistry, covalent interactions with target proteins can be captured and characterized. This strategy is instrumental in elucidating drug mechanisms of action and assessing potential off-target effects.
3. Universal Labeling Based on Reactive Residues
Universal reactive groups (e.g., IA-alkyne for labeling cysteine residues) are used to label accessible and reactive sites on protein surfaces. This approach is particularly suited for investigating changes in residue accessibility caused by conformational alterations or ligand binding. It is also applicable to protein stability studies, including thermal shift assays.
4. Chemical Labeling Combined with Quantitative Mass Spectrometry (Chemical Quantitative Proteomics)
By integrating with quantitative proteomic techniques such as SILAC, TMT, and iTRAQ, this strategy enables relative or absolute quantification of protein modifications under various treatment conditions. It supports time-course experiments and comparative analyses across multiple experimental groups, and is widely used to study dynamic biological processes such as drug treatment, stress responses, and metabolic perturbations.
Advantages and Features of Chemical Proteomics
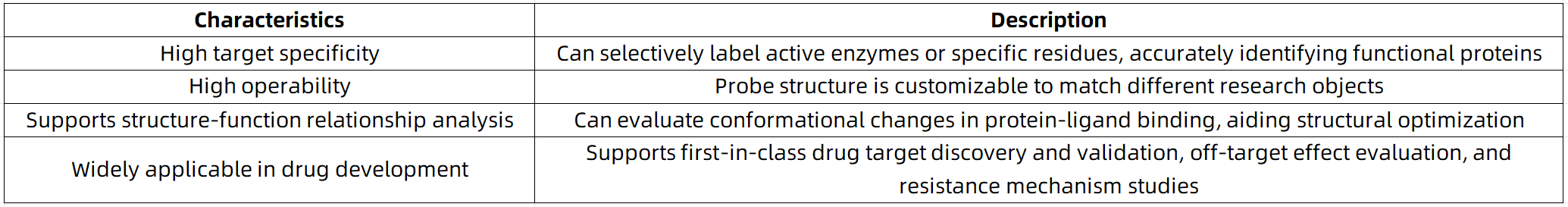
Typical Application Scenarios
1. First-in-class Drug Target Identification
For novel small-molecule compounds that lack the typical features of classical drug targets, chemical proteomics combined with covalent probes enables the direct identification of target proteins, uncovering previously unrecognized functional interfaces of otherwise undruggable proteins.
2. Target Validation and Off-target Assessment of Covalent Inhibitors
Chemical proteomics allows direct profiling of covalent interactions between small molecules and proteins, facilitating the evaluation of potential toxicity risks and the selection of molecular structures with improved specificity.
3. Functional Proteome and Active Enzyme Landscape Mapping
Comprehensive activity-based profiling of key enzyme families, such as esterases and proteases, across different tissues and pathological conditions enables global insight into their dynamic regulation and functional states.
4. Dissecting Small Molecule-induced Conformational Changes and Residue Accessibility
Alterations in protein modification patterns can be leveraged to infer small molecule-induced conformational rearrangements and to predict potential regulatory sites, supporting fine-tuned modulation of protein function.
MtoZ Biolabs offers end-to-end services encompassing probe design, reaction condition optimization, protein enrichment, mass spectrometry-based analysis, and data interpretation:
Chemical proteomics offers a precise and systematic approach to studying protein function and elucidating drug mechanisms in the post-genomic era. By integrating selective labeling strategies with advanced mass spectrometry, this platform enables in-depth characterization of protein activity and interaction dynamics. It has found broad applications in cutting-edge research and drug development across oncology, immunology, and metabolic diseases. Looking ahead, with advancements in probe diversity, quantitative methodologies, and AI-assisted data interpretation, chemical proteomics is poised to play an increasingly pivotal role in precision medicine and innovative drug discovery.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







