Biopharmaceutical Variation Analysis Service
In biopharmaceuticals, the therapeutic effectiveness, stability, safety, and pharmacokinetic attributes of drugs are critically influenced by proteins' post-translational modifications and sequence variations. The inherent complexity of biopharmaceuticals demands sophisticated analytical techniques to guarantee that each production batch adheres to stringent quality standards. Variation analysis plays a crucial role in evaluating and ensuring the structural integrity and functionality of biopharmaceuticals, particularly during the development and approval stages of biosimilars.
MtoZ Biolabs offers advanced variation analysis services for biopharmaceuticals, utilizing cutting-edge mass spectrometry to precisely identify minute variations in drug molecules. This service aims to refine product design, expedite clinical trials, and ensure the efficacy and safety of biopharmaceuticals.
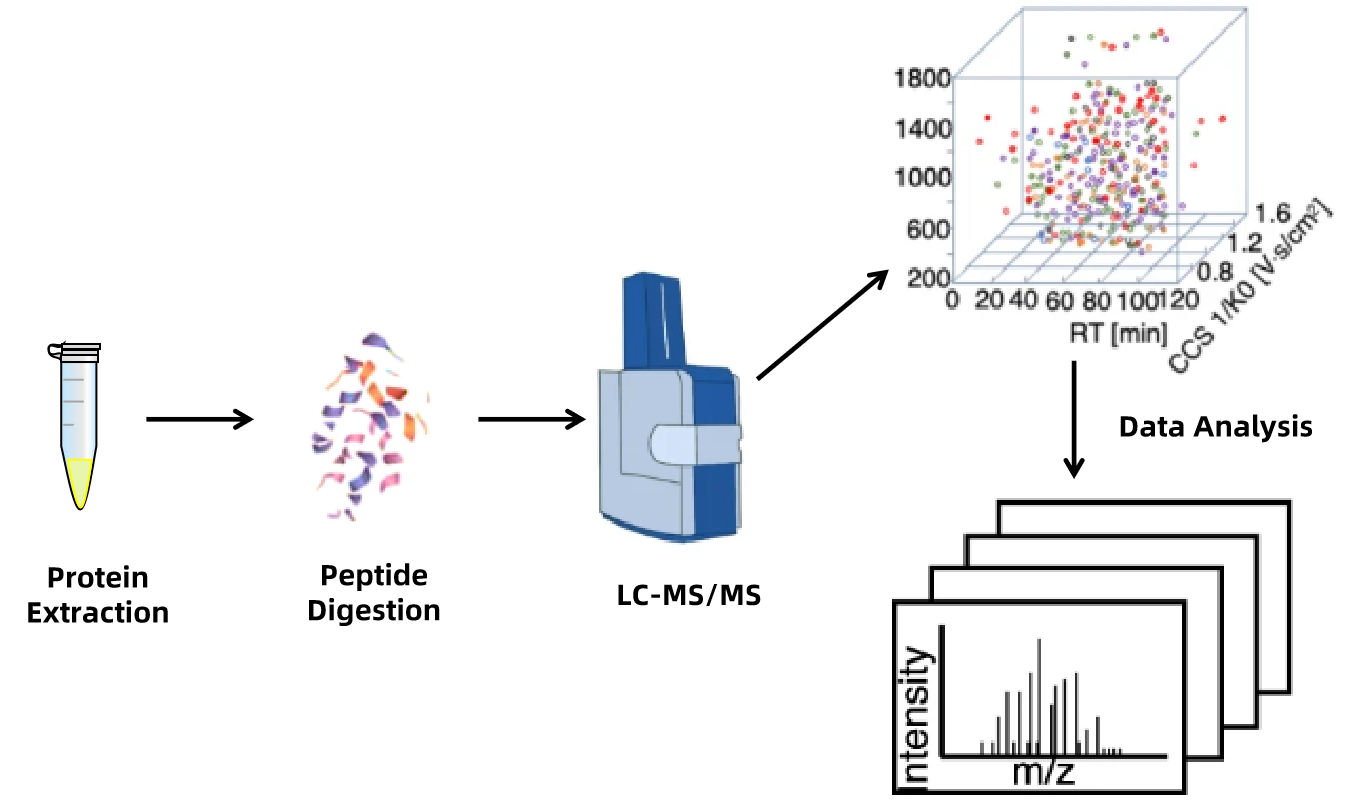
Figure 1. Biopharmaceutical Variation Analysis Workflow
Services at MtoZ Biolabs
· Biopharmaceutical Disulfide Bond Analysis Service
· Biopharmaceutical Glycan Analysis Service
· Biopharmaceutical Glycosylation Site Analysis Service
· Antibody C-terminal Variation Analysis Service
· Antibody N-terminal Cyclization Analysis Service
· Antibody Deamidation and Isomerization Analysis Service
Experimental Instruments
Liquid Chromatography-Mass Spectrometry (LC-MS): Equipped with advanced instruments such as the Thermo Fisher Orbitrap mass spectrometer, this technology delivers ultra-high resolution and rapid scanning capabilities.
Service Advantages
High Precision and Sensitivity: Capable of detecting variations at extremely low abundances, ensuring the precision and reliability of our analysis.
Broad Applicability: Applicable to all types of biopharmaceuticals, including antibodies, vaccines, and fusion proteins.
Rapid Response and Efficiency: Our standard analysis workflow delivers results within a week.
Customized Service: Tailored solutions designed to meet the diverse needs of our clients.
Applications
1. Drug Development
In the early phases of drug development, variation analysis is employed to optimize the molecular structure of candidate drugs, enhancing their efficacy and safety.
2. Quality Control
Ensures the stability and consistency of biopharmaceutical products through regular monitoring during production.
3. Market Regulation
Continuous quality monitoring of drugs post-market to maintain long-term therapeutic effectiveness and safety.
4. Biosimilar Comparison
Evaluates and verifies the structural and functional similarities between biosimilars and original drugs.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Biopharmaceutical Glycan Analysis Service
How to order?







