Biomarker Validation Service
- High Precision and Sensitivity: Our advanced technology platform ensures precise detection of biomarkers, even at low abundance or with subtle differences.
- Multidimensional Analysis: Combining genomics, proteomics, and conventional molecular biology techniques, we provide comprehensive biomarker validation.
- Customized Services: Tailored experimental plans are designed to meet each client's specific research needs, ensuring that biomarker validation aligns with research objectives.
- High Throughput and Efficiency: With state-of-the-art high-throughput technology, we can analyze multiple biomarkers simultaneously, improving efficiency and data output.
Biomarker validation refers to the rigorous confirmation of the performance and reliability of specific molecules or characteristics across various biological contexts using standardized experimental methods. The process of biomarker validation not only aims to verify the biological function of biomarkers under particular conditions but also assesses their reproducibility, sensitivity, specificity, and quantitative precision in experimental settings. With the rapid advancement of precision medicine and personalized treatments, biomarkers hold substantial promise for early disease screening, prognosis evaluation, and monitoring therapeutic responses. However, initial discoveries alone are insufficient for clinical or research application; strict experimental validation is essential to ensure their reliability and effectiveness.
MtoZ Biolabs is dedicated to providing high-quality Biomarker Validation Service to research institutions, pharmaceutical companies, and biotechnology firms. Our service span from basic research to drug development, supporting clients in validating potential biomarkers in laboratory environments and advancing their translation into practical applications.
Services at MtoZ Biolabs
MtoZ Biolabs offers a range of established laboratory biomarker validation methods to ensure that each biomarker is accurately and reproducibly validated under varying experimental conditions. Our approaches encompass not only traditional molecular biology techniques but also advanced omics technologies, offering multidimensional validation support to our clients.
1. qPCR/dPCR
qPCR and dPCR are widely utilized gene expression quantification technologies in biomarker validation. qPCR provides high-sensitivity quantitative data by monitoring fluorescence signals in real-time during the PCR reaction, making it ideal for detecting low-abundance biomarkers. In contrast, dPCR employs digital molecular amplification technology to enhance quantitative precision and ensure highly reliable detection results.
2. Top-Down Proteomics
Top-down proteomics analyzes intact protein molecules directly, rather than fragmented peptides, offering a comprehensive overview of the protein. This approach is vital for validating proteins or their modifications associated with diseases. Using mass spectrometry, we can precisely identify structural features and functional properties of target proteins, confirming their potential as biomarkers.
3. Olink Proteomics
The Olink proteomics platform combines immunocapture-based targeted protein detection with highly sensitive mass spectrometry to simultaneously analyze the expression levels of hundreds of target proteins. This method is particularly suited for validating complex biomarker panels, offering high-throughput, precise data to help uncover disease-associated proteins.
4. Other Validation Methods
In addition to these advanced techniques, MtoZ Biolabs offers traditional protein analysis methods such as ELISA and Western Blot, widely used for protein quantification and validation. We provide flexible, customized biomarker validation solutions by integrating different methods based on client needs.
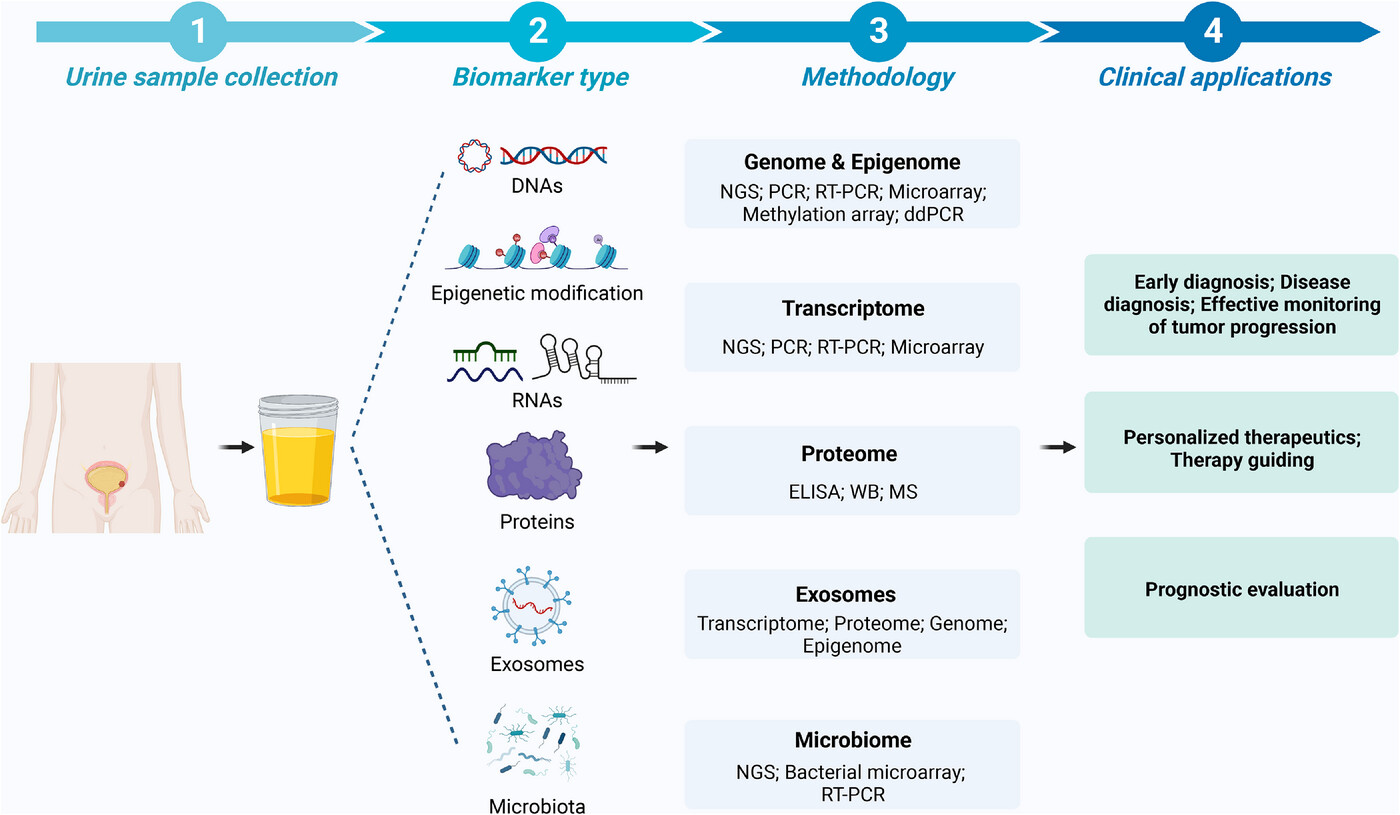
Zeng, Y. C. et al. CTD. 2023.
Figure 1. The Workflow for Biomarker Validation
Service Advantages
MtoZ Biolabs' biomarker validation service offers several key technical advantages:
Case Study
Case 1: LC-MS/MS Technology for Accurate Validation of ECG Biomarkers
This study utilized LC-MS/MS to develop a highly sensitive and specific method for quantifying the hERG channel blocker Dofetilide in human plasma, achieving a detection limit at the picomolar level. This approach was successfully employed in Phase I clinical trials to validate the correlation between Dofetilide and electrocardiogram (ECG) biomarkers. By establishing connections between pharmacokinetics (PK) and pharmacodynamics (PD), this research provides scientific evidence for the role of ECG biomarkers in drug safety assessments, highlighting the value of biomarker validation service in clinical research and drug development.
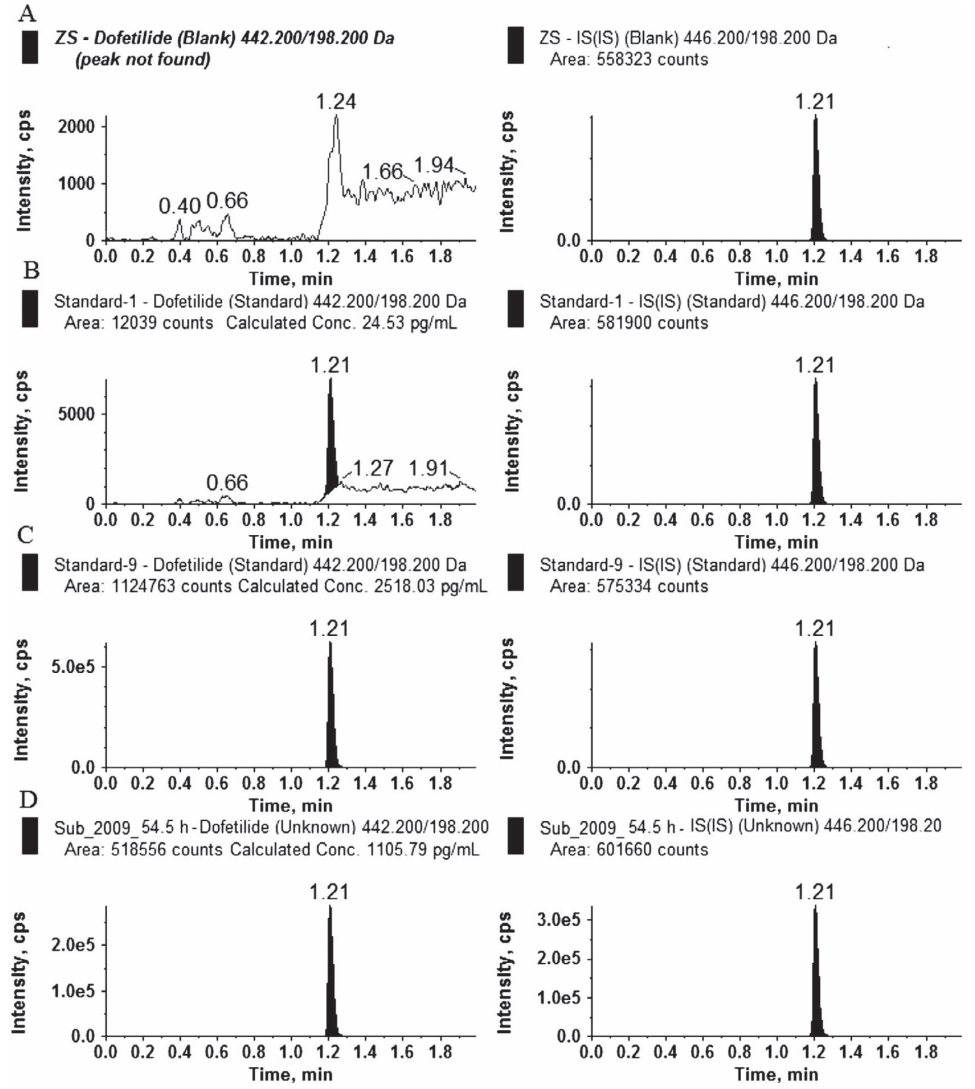
Matta, M. K. et al. J Anal Toxicol. 2020.
Case 2: UPLC-MS/MS Technology for Accurate Validation of Metabolic Disease Biomarkers
This study employed UPLC-MS/MS to detect and quantify specific oligosaccharides and glycosaminoglycans in urine, successfully identifying potential biomarkers related to mucopolysaccharidosis (MPS) and glycoproteinosis. Through rigorous validation, the study confirmed the significant association of these molecules with disease states, demonstrating their reliability and potential as diagnostic tools. This case showcases the essential role of biomarker validation service in metabolic disease biomarker discovery, providing scientific evidence and technical support for the accurate diagnosis of genetic metabolic disorders.
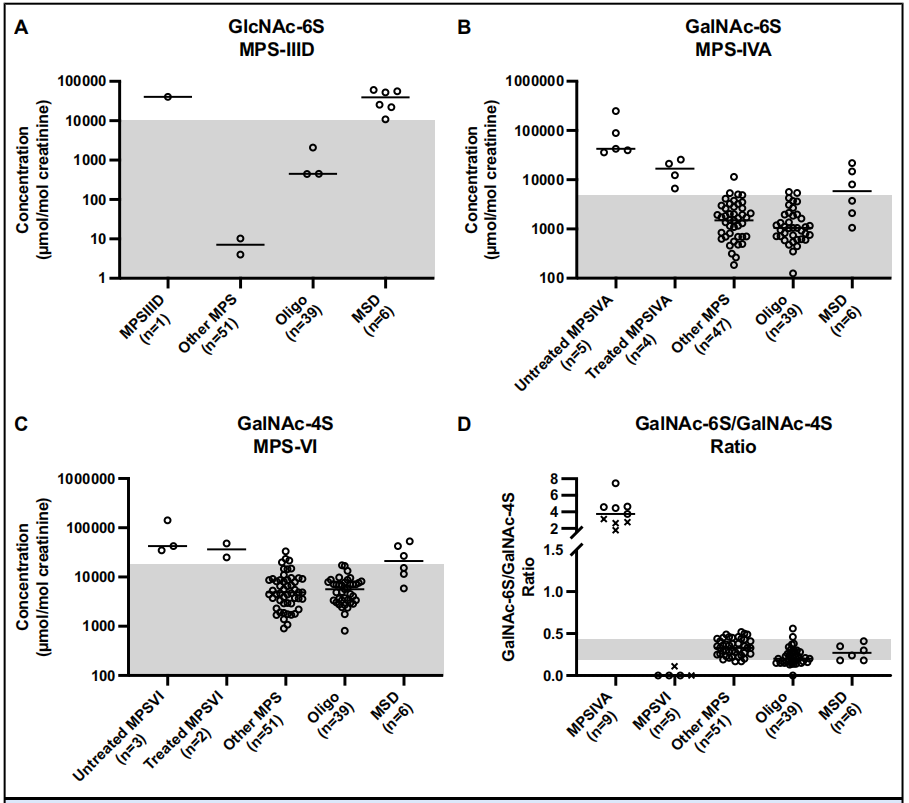
Wongkittichote, P. et al. Clin. Chem. 2024.
MtoZ Biolabs' biomarker validation service is designed to provide precise and efficient data support for researchers and pharmaceutical companies. We focus on oncology, neuroscience, immunology, metabolic diseases, and other areas, helping clients validate and screen potential biomarkers. During drug development, our services provide strong data support for new drug discovery and contribute to disease mechanism research, early diagnosis, and prognosis evaluation. Through MtoZ Biolabs' biomarker validation service, clients can advance biomarker research more swiftly and accurately, ensuring the reliability of experimental data and offering essential support for preclinical trials and future clinical applications.
How to order?







