Bioactive Peptide Component Identification and Quantification
Bioactive peptides are short protein fragments of 2-50 amino acids in length that are linked by covalent bonds (called amide bonds or peptide bonds). Bioactive peptides have the advantages of simple structure, high biological activity, high specificity and low toxicity, and have broad prospects in the design and development of new peptide drugs.
Bioactive peptides have a variety of biological functions:
1. Antimicrobial Peptides
Antimicrobial peptides, also known as host defense peptides, have broad-spectrum antibacterial and immunomodulatory activities against infectious bacteria (Gram-positive and Gram-negative), viruses, and fungi. Antimicrobial peptides can treat infections and are also effective broad-spectrum antibiotics. More than 2000 antimicrobial peptides have been identified from various organisms (animals, fungi, plants and bacteria).
2. Antioxidant Active Peptides
The antioxidant activity of bioactive peptides can be attributed to their ability of free radical scavenging, inhibition of lipid peroxidation, and chelation with metal ions. Oxidative damage can affect the health of the body by altering the structure and function of cells. Antioxidant peptides have the effect of inhibiting and delaying lipid oxidation, and can protect human tissues and organs from free radicals.
3. Anticancer Active Peptides
Anticancer active peptides can inhibit the proliferation or metastasis of tumor cells, or inhibit the formation of tumor blood vessels, and has less drug resistance than traditional chemotherapy drugs. Such as melitin, Arg-Gly-Asp (RGD) peptides, etc. However, anticancer active peptides may be degraded by proteases, causing cytotoxicity. Thus, a large number of studies in recent years have focused on the reconstruction or structural modification of anticancer active peptides to improve their anticancer activity and reduce their cytotoxicity.
4. Immune Peptides
Immune peptides play an immunomodulatory role. For example, thymopentin (TP-5), a synthetic drug, and muramyl dipeptide (MDP), which can strengthen cellular immune response and activate macrophages, etc., are mainly used for immunocompromised pneumonia, cancer and other diseases. In addition, bioactive peptides have a variety of other activities, such as antithrombin, reducing high blood pressure and blood lipids, anti-fatigue and preventing and treating osteoporosis, etc.
Peptides are present in almost all living organisms and have a variety of important roles in the body. Peptides are often easily synthesized by chemical pathways or recombination. So they are promising reagents for the treatment and diagnosis of diseases as well as ingredients for the fabrication of bionanomaterials.
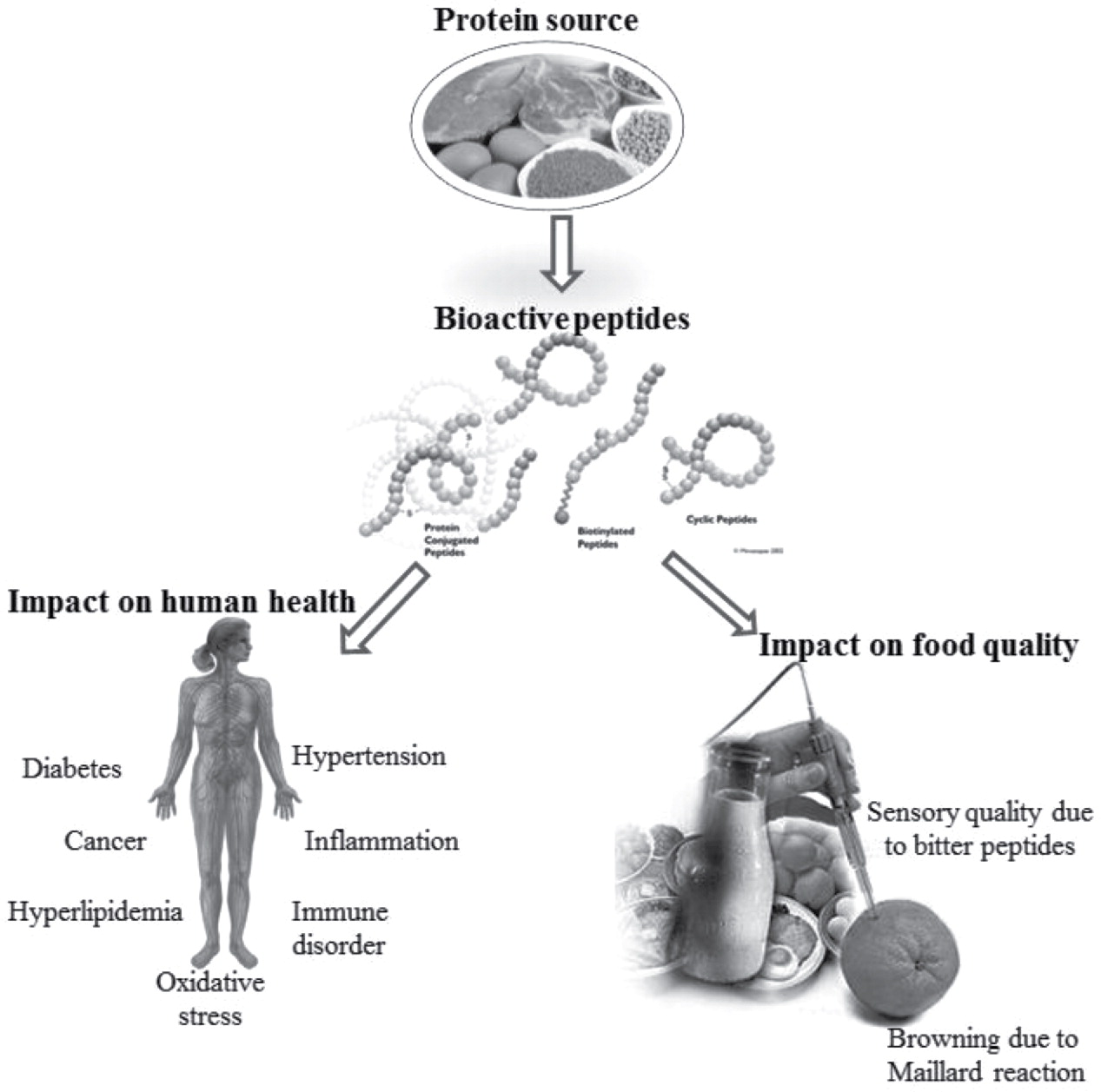
Figure 1. Bioactive Peptides and Their Effects on Human Health[1]
The process of identification and quantification of peptide active ingredients can be divided into extraction, separation, purification and identification analysis of active peptides.
1. The extraction methods of bioactive peptides mainly include:
(1) Enzymatic Hydrolysis Method, in which specific proteases are selected for enzymatic modification and enzymatic hydrolysis of proteins into peptides or amino acids, is a common and easy-to-control method. At present, the single enzyme hydrolysis and the compound enzyme hydrolysis methods composed of two or more proteases are widely used.
(2) Chemical Synthesis Method, the principle of which is the condensation reaction of amino acids from the C-terminal to the N-terminus, currently includes liquid phase synthesis method, solid phase synthesis method, native chemical ligation method, etc.
(3) Genetic Engineering Synthesis Method, which uses DNA recombinant technology, Escherichia coli and other engineered bacteria to produce polypeptides on a large scale.
(4) Microbial Fermentation Method, under specific culture conditions, microorganisms can produce proteases through metabolism to degrade proteins in the environment to produce small molecule polypeptides.
2. The separation and purification methods of bioactive peptides mainly include:
(1) Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC), which refers to the elution chromatography method of solute separation and purification according to the polarity difference of samples (hydrophobicity) by using a non-polar reversed-phase medium as the stationary phase and the aqueous solution of the polar organic solvent as the mobile phase. RP-HPLC can analyze target peptides in batches with high resolution, sensitivity and good separation effect.
(2) Gel Filtration Chromatography (GFC), which is a technique for separating molecules of different sizes according to the blocking effect of the packed gel, and different components will be separated due to their different particle sizes, different moving paths, and different elution times.
(3) Ion-Exchange Chromatography (IEC), which is a column chromatography method that uses the different charge forces between the exchangeable ions on the ion exchanger and various charged ions in the surrounding medium to achieves the purpose of separation.
(4) Electrophoresis Technology, in which proteins and peptides exhibit different electrophoresis under the action of electric field and then are separated by different charges and molecular weights of proteins and peptides.
(5) Affinity Chromatography, which uses one of the two substances with a high degree of specific affinity with each other as the stationary phase, and uses different degrees of affinity with the stationary phase to separate the components with impurities.
(6) Ultrafiltration Technology, which uses the pressure difference between the two ends of the membrane to separate proteins and peptides of 1-500 kDa, is an effective method for separating small molecules.
(7) Aqueous Two-Phase Extraction Technology, which separates substances with different partition coefficients in the immiscible aqueous phase.
In addition to the application of a single separation method, multiple separation methods, such as IEC/RP-LC, can also be combined to achieve better separation results.
3. The identification and analysis methods of bioactive peptides mainly include:
(1) Mass Spectrometry (MS), in which the mass of the molecular ions and fragments of the sample ionized is determined, and the relative molecular mass and molecular structure of the sample are determined. MS can not only detect the molecular weight information of polypeptides, but also be used to show their primary structure with high throughput and resolution.
(2) Edman Degradation Method, a method to analyze the composition and amino acids arrangement of peptides from the N-terminus by using the modification of phenyl isothiocyanate.
(3) Ultraviolet/Infrared Spectroscopy, which can infer the possible groups in multiple peptides through the stretching vibration of the characteristic group. This method is mainly used to analyze the groups and secondary structures of peptides.
(4) Circular Dichroism (CD), which can identify and predict the secondary structure of peptides.
(5) Nuclear Magnetic Resonance (NRM), usually coupled with infrared spectroscopy to identify special functional groups in peptides, generally be used to analyze peptide with less than 30 amino acids.
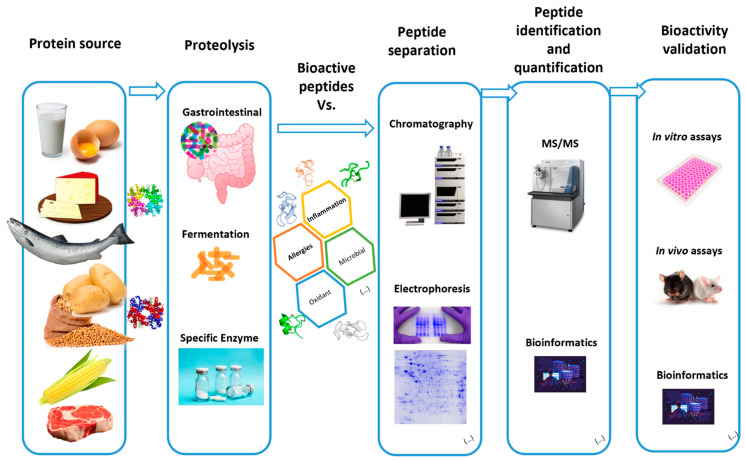
Figure 2. Methods for Extraction, Isolation and Identification of Active Peptides [2]
Analysis Workflow
1. Extract Peptides
2. Isolate and Purify Peptides
3. Peptide Identification and Quantification
4. Data Analysis
Service Advantages
1. Identification and Quantification of Peptides from Multiple Types of Sample Sources
2. High credibility, High-Precision MS Detection
3. Comprehensive Bioinformatics Analysis
Sample Results
1. Preparation and Identification of Novel Antioxidant Peptides in Camel Bone Protein [3]
This study aimes to prepare collagen with antioxidant activity by hydrolyzing camel bone and to identify the peptides having antioxidant activity. Firstly, single factor plus orthogonal experiments were carried out to optimal preparation conditions. The determined hydrolysis time was 5 h, the enzyme-bottom ratio at 1200 U/g, the pH at 7.0, and the material-water ratio at 1:3.0. Subsequently, the hydrolysate was purified through a series of chromatographic procedures. GPPGGPPGPPGPPSGGFDF (hydroxylated), PATGDLTDFLK, and GSPGPQGPPGSIGPQ, the three novel peptides with antioxidant activity were identified from the fractions using liquid chromatography-tandem MS. The peptide PATGDLTDFLK exhibits excellent DPPH free radical scavenging activity (39%) and can increase 21.1% antioxidant activity of damaged HepG 2 cells induced by H2O2.
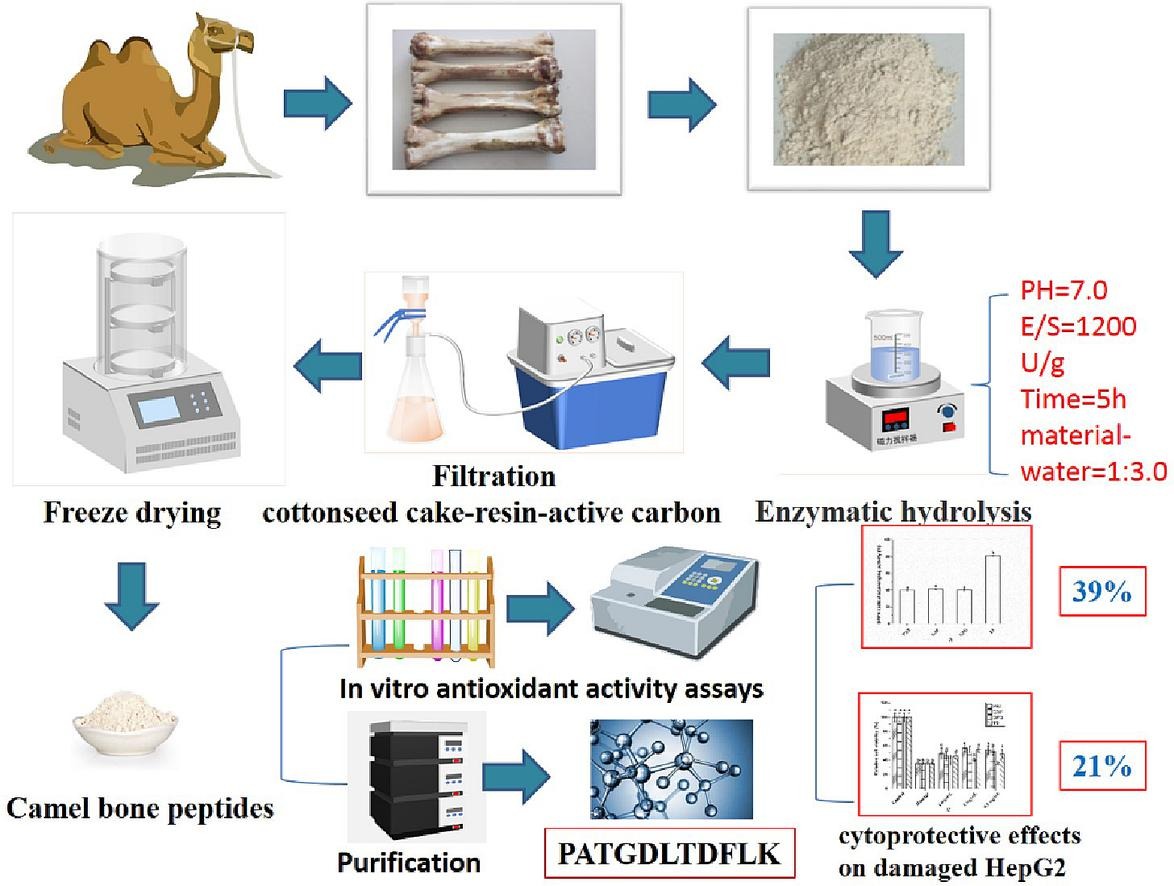
Figure 3. Novel Antioxidant Peptide Identification Process in Camel Bone Protein
2. Identification of NF-κB Inhibitory Peptides from Tryptic β-Casein Hydrolysates [4]
This study aims to identify the potential anti-inflammatory activity of the hydrolysate of bovine β casein (β-CN) after enzymatic hydrolysis by trypsin. β-CN was hydrolyzed by trypsin and fractionated by ultrafiltration combined with HPLC. Total protein hydrolysates, as well as fractions containing peptides from 1 to 5 kDa, showed inhibition in cell-based assays, while fractions containing molecules less than 1 kDa did not. This anti-inflammatory effect is attributed to a large group of hydrophobic peptides that were identified with LC-MS. The main peptide was synthesized in vitro and showed significant anti-inflammatory effects.
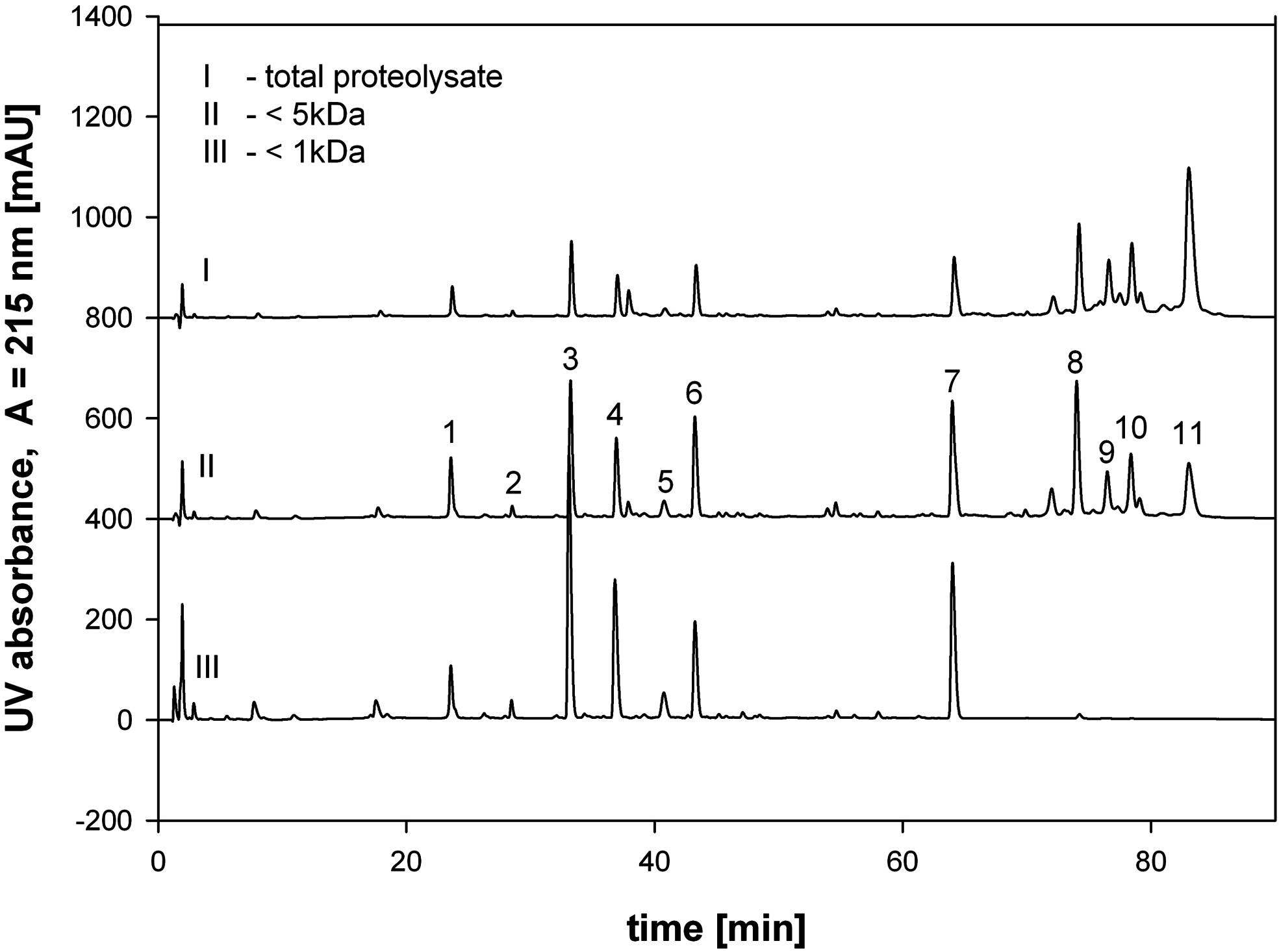
Figure 4. Preparation of Tryptic Peptide Fractions
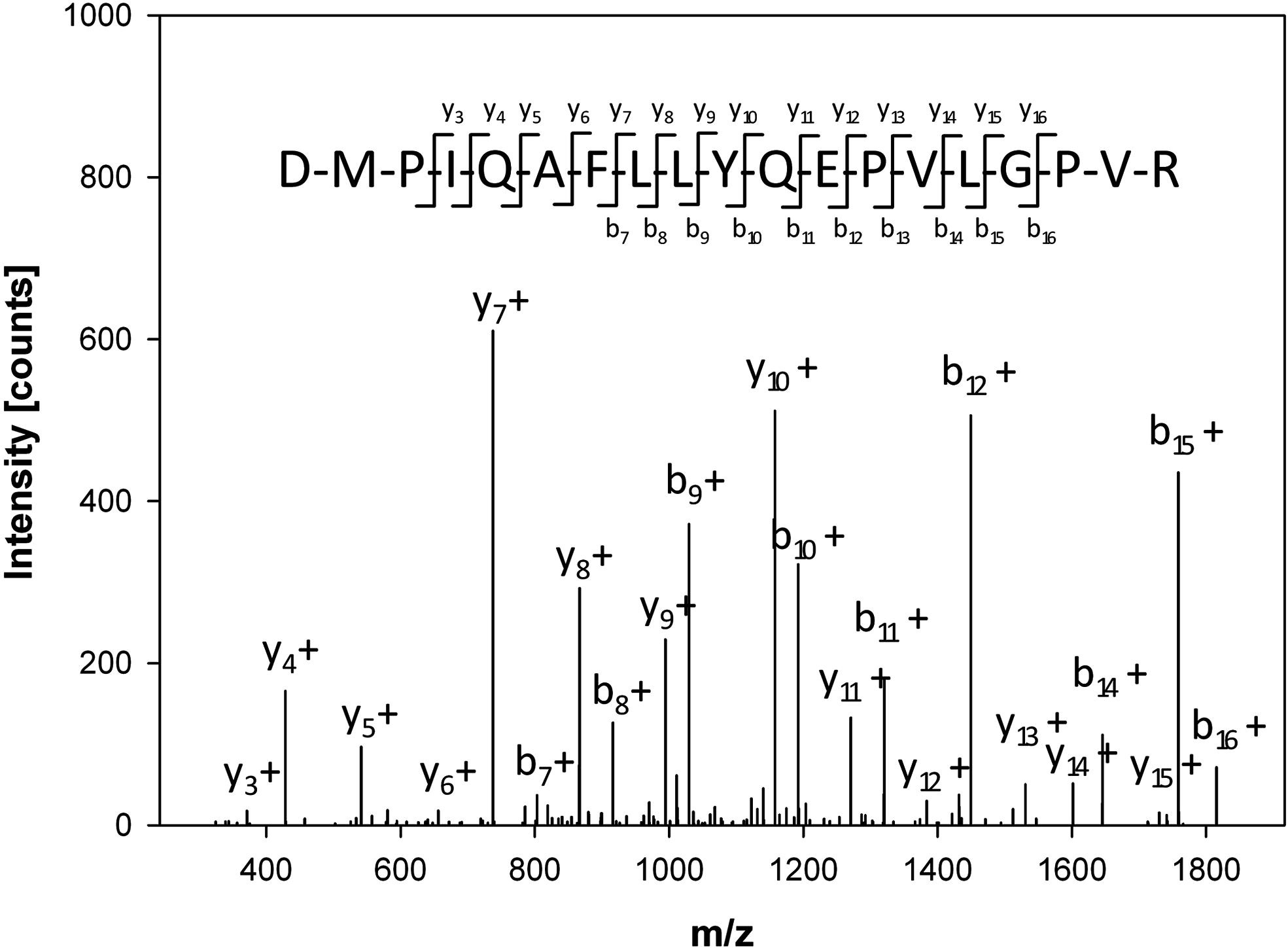
Figure 5. Sequence Identification of Peptides
Sample Submission Requirements
1. Provide treated purified samples whenever possible.
2. Try to avoid impurity pollution.
Services at MtoZ Biolabs
1. Complete Experimental Protocol
2. Relevant Instrument Parameters
3. Raw MS Data
4. Detailed Information Report on the Identification of Peptides
Applications
1. Peptide Growth Factor
It can strongly promote cell growth and have a regulatory effect on cell differentiation, growth, proliferation and function. Such as epidermal growth factor (EGF), insulin-like growth factor (IGF-I), interleukin-converting growth factor (TGF-1), nerve growth factor (NGF), etc., among which EGF has been widely used in skin diseases, beauty, skin beautification and other fields.
2. Cardiovascular Active Peptides
They include antihypertensive peptides, and anti-atrial fibrillation peptides containing angiotensin-converting enzyme (ACE), as well as atrial natriuretic peptide, B-type brain natriuretic peptide, endothelial vasoconstrictor peptide, relaxin, and mediatin, etc.
3. Anticancer Active Peptides
They contain bee venom peptides, RGD peptides, etc.
4. Food Peptides
They exist in food, dietary supplements, and special medical food. It is easy to digest and absorb, such as soybean polypeptide.
5. Antioxidant Peptides
They can eliminate superoxide radicals, hydroxyl radicals and alkane radicals, etc. They are glutathione, soybean polypeptides, corn gluten peptides, wheat protein-derived antioxidant peptides, rice bran protein-derived antioxidant peptides, dairy antioxidant peptides, and marine biological antioxidant peptides.
6. Antihypertensive Peptides
ACE, soybean polypeptide, corn gluten peptide, etc.
7. Hypolipidemic and Cholesterol Peptides
Ginseng peptides, jellyfish peptides, soybean peptides, etc.
8. Immunomodulatory Peptides
Thymus peptides, spleen peptides, sheep placenta peptides, etc.
9. Flavoring Peptides (to improve feed flavor)
Including sour, sweet, bitter, salty, such as sweetener, alitamine, etc.
FAQ
Q1: There are many methods for the preparation, separation and identification of bioactive peptides, which one is more commonly used?
For the extraction of bioactive peptides, the most commonly used method is enzymatic hydrolysis, because it is easy to operate and the enzymatic digestion results are easy to analyze. For peptide separation, reversed-phase chromatography is commonly used, which has the characteristics of high resolution, good separation effect, and batch analysis of target peptides. For peptide identification, MS is the most commonly used method, which has the advantages of high throughput and high resolution.
References
[1] Daliri EB, Lee BH, Oh DH. Current trends and perspectives of bioactive peptides. Crit Rev Food Sci Nutr. 2018;58(13):2273-2284. doi: 10.1080/10408398.2017. 1319795. Epub 2017 Sep 29. PMID: 28604060.
[2] Abril AG, Pazos M, Villa TG, Calo-Mata P, Barros-Velázquez J, Carrera M. Proteomics Characterization of Food-Derived Bioactive Peptides with Anti-Allergic and Anti-Inflammatory Properties. Nutrients. 2022 Oct 20;14(20):4400. doi: 10.3390/nu14204400. PMID: 36297084; PMCID: PMC9609859.
[3] Wang J, Yang G, Li H, Zhang T, Sun D, Peng Lu W, Zhang W, Wang Y, Ma M, Cao X, Zhang B, Guo Y. Preparation and identification of novel antioxidant peptides from camel bone protein. Food Chem. 2023 Oct 30;424:136253. doi: 10.1016/j.foodchem.2023.136253. Epub 2023 May 12. PMID: 37236074.
[4] Malinowski J, Klempt M, Clawin-Rädecker I, Lorenzen PC, Meisel H. Identification of a NFκB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014 Dec 15;165:129-33. doi: 10.1016/j.foodchem.2014.05.075. Epub 2014 May 23. PMID: 25038658.
How to order?







