Antibody De Novo Sequencing Service
Antibody de novo sequencing is a cutting-edge approach for determining the complete amino acid sequence of antibodies without prior sequence knowledge. This method uses advanced mass spectrometry combined with bioinformatics to reconstruct both heavy and light chain sequences with high precision. Antibody de novo sequencing is particularly essential for characterizing antibodies when sequence information is unavailable, such as for hybridoma-derived antibodies, therapeutic antibodies, or proprietary antibodies lacking sequence records.
Antibody de novo sequencing addresses key challenges in antibody research and development, including verifying sequence accuracy, identifying post-translational modifications, and enabling the production of biosimilars. By providing precise sequence data, antibody de novo sequencing ensures batch-to-batch consistency, and facilitates antibody engineering for enhanced efficacy and stability. This technology is indispensable for advancing therapeutic antibody development and innovation.
Services at MtoZ Biolabs
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. MtoZ Biolabs provides a highly accurate antibody de novo sequencing service that determines the complete amino acid sequence of monoclonal and polyclonal antibodies without the need for reference databases. By combining advanced mass spectrometry technology with proprietary bioinformatics algorithms, we can precisely identify the heavy and light chains of antibodies, including variable and constant regions. Our antibody de novo sequencing service covers hybridoma sequencing, antibody humanization, and therapeutic antibody development, supporting a wide range of applications from biomedical research to biopharmaceutical production. MtoZ Biolabs is dedicated to delivering reliable data to accelerate antibody innovation. If you are interested in our services, please feel free to contact us.
Analysis Workflow
Our antibody de novo sequencing service offers but not is limited to the following approaches, with specific methods tailored to the sample type and research objectives:
1. Recombinant Antibody Sequencing: Utilizing mass spectrometry (e.g., the shotgun approach) to directly sequence recombinant antibodies at the peptide level, reconstructing the complete amino acid sequence. Through data analysis and sequence assembly, a full de novo sequence is obtained.
2. Endogenous Antibody Repertoire Analysis: Combining shotgun mass spectrometry with RNA sequencing (RNA-seq) to extract antibody-encoding information from B cells and perform proteomics database searches. This approach typically achieves partial de novo sequencing and is used to analyze the diversity of endogenous antibodies.
3. Endogenous Antibody Sequencing: Integrating shotgun and middle-down mass spectrometry techniques through hybrid MS to analyze endogenous antibodies and perform sequence assembly. This method allows for partial or complete de novo sequencing and is suitable for complex endogenous antibody samples.
By flexibly applying these methods, antibody de novo sequencing service provides high-accuracy sequence information to meet diverse research and application needs.
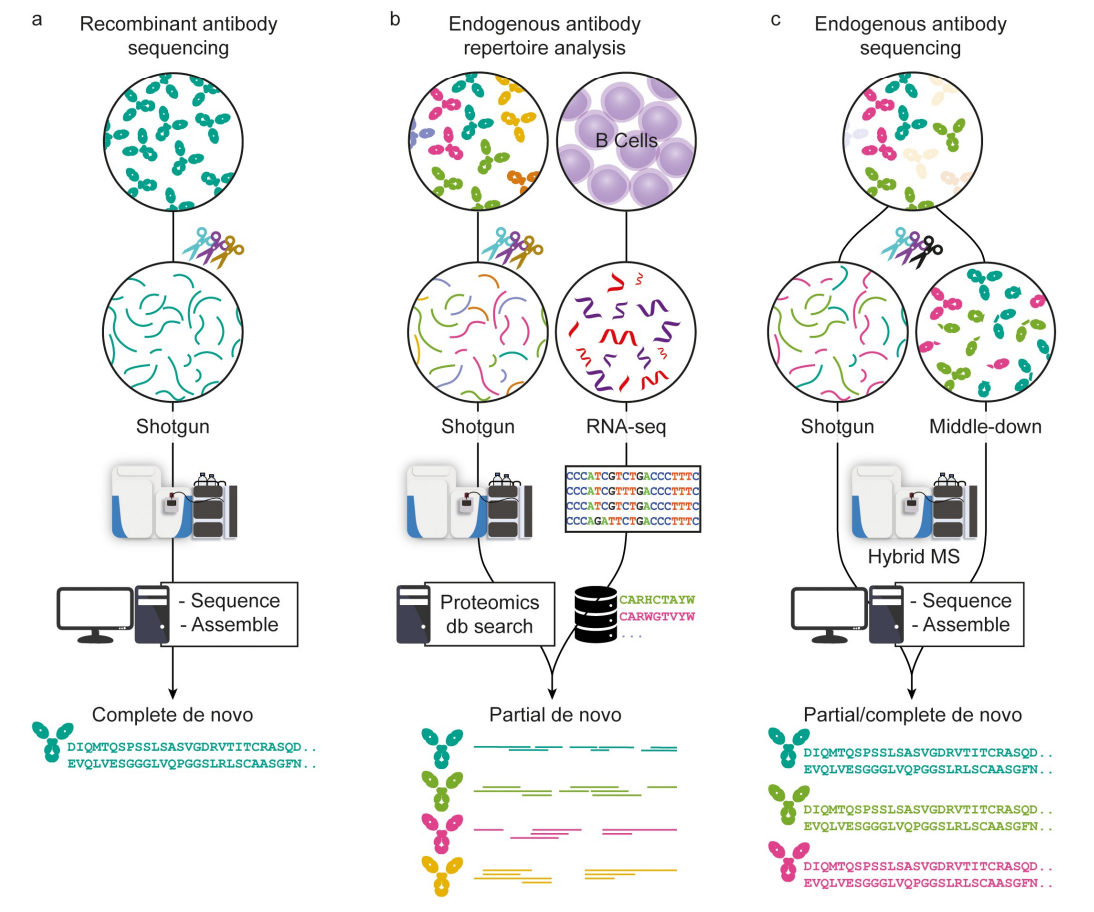
De, Graaf, SC. et al. MAbs. 2022.
Figure 1. The Workflow of Antibody De Novo Sequencing
Service Advantages
1. High Accuracy and Coverage
MtoZ Biolabs' antibody de novo sequencing service combines advanced technologies and intelligent algorithms to ensure high accuracy and comprehensive sequence coverage.
2. Optimized Solutions for Heavy and Light Chains
Our antibody de novo sequencing service employs flexible strategies to address the challenges of heavy chain sequencing while delivering exceptional performance for light chain analysis.
3. Robust Error Correction and Data Analysis
The antibody de novo sequencing service utilizes efficient error correction and data processing workflows to provide reliable sequence analysis results, meeting the demands of complex samples.
Case Study
1. De Novo Protein Sequencing of Antibodies for Identification of Neutralizing Antibodies in Human Plasma Post SARS-CoV-2 Vaccination
This study presents a method combining mass spectrometry and B-cell sequencing to analyze human plasma-derived polyclonal IgG antibodies in response to the Moderna Spikevax COVID-19 vaccine. By sequencing the natural polyclonal response, 12 recombinant antibodies were generated, including six with de novo protein sequencing. Among them, four de novo-sequenced antibodies demonstrated equal or superior binding affinities compared to natural polyclonal antibodies. Neutralization tests confirmed their neutralizing capabilities against the target antigen. This approach highlights the importance of directly examining the circulating IgG pool, which may not be fully represented by B-cell analysis alone. Antibody de novo sequencing service utilizes advanced mass spectrometry to decode antibody sequences directly from plasma, enabling the identification and generation of recombinant antibodies with strong binding affinities and neutralizing capabilities. This service supports precise antibody characterization and optimization.
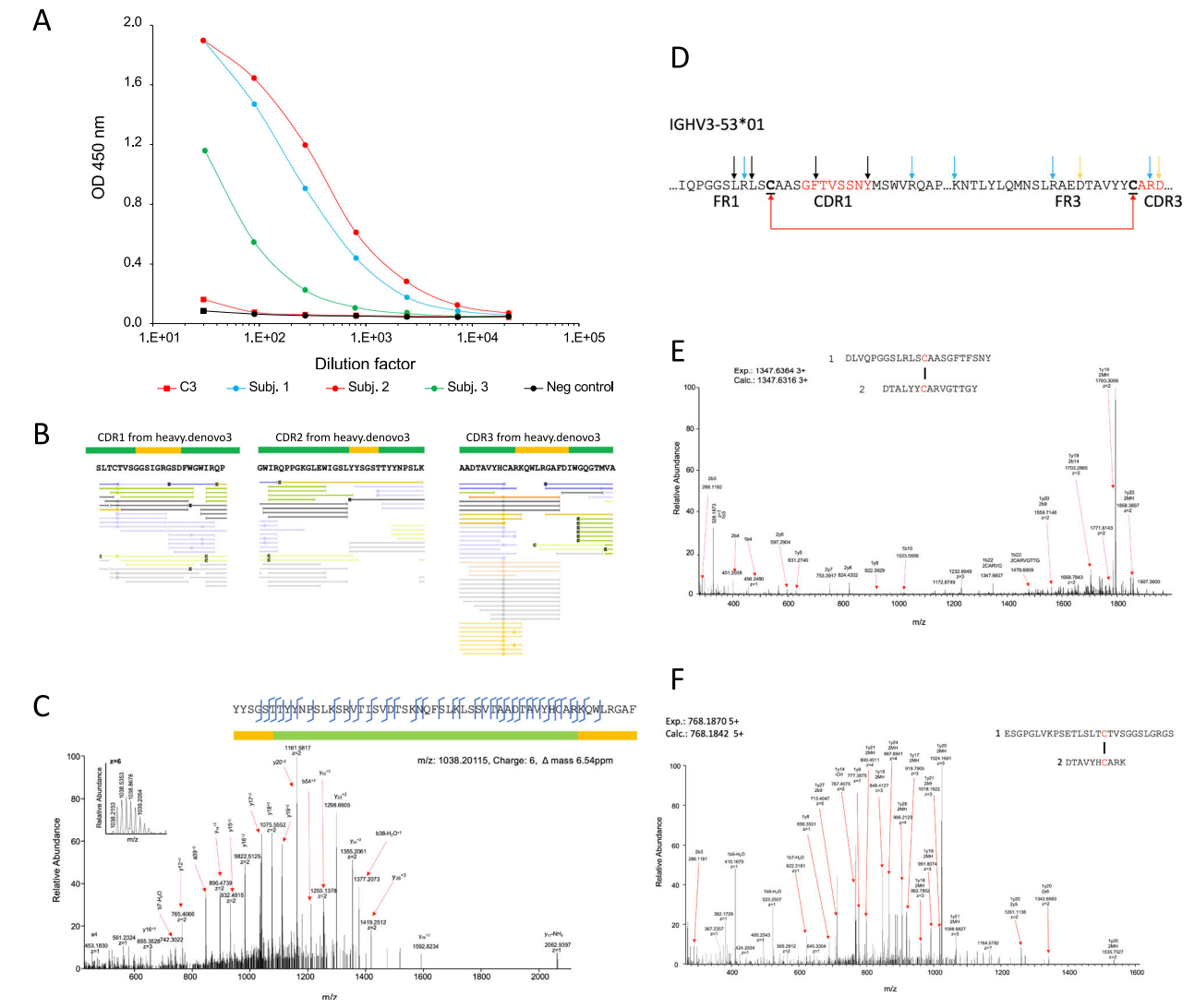
Le, Bihan, T. et al. Nat Commun. 2024.
Figure 2. MS-Based Antibody De Novo Sequencing
2. Mass Spectrometry-Based De Novo Sequencing of Endogenous Antibodies
This article explores the advancements and challenges of mass spectrometry (MS)-based de novo sequencing in the study of recombinant and endogenous humoral antibodies. Traditional methods rely on nucleotide sequencing of B-cell receptor (BCR) repertoires, while MS enables direct sequencing at the protein level, revealing critical structural information about antibodies. The study highlights the specific challenges of analyzing endogenous antibodies and complex clinical samples (e.g., serum), which require tailored approaches beyond conventional proteomics workflows. The article reviews recent progress in MS techniques, demonstrating significant strides in overcoming these obstacles and expanding the feasibility of de novo antibody sequencing. Antibody de novo sequencing service employs advanced mass spectrometry techniques to directly determine the amino acid sequences of antibodies. This service addresses the challenges posed by complex clinical samples, offering customized solutions for precise antibody characterization.
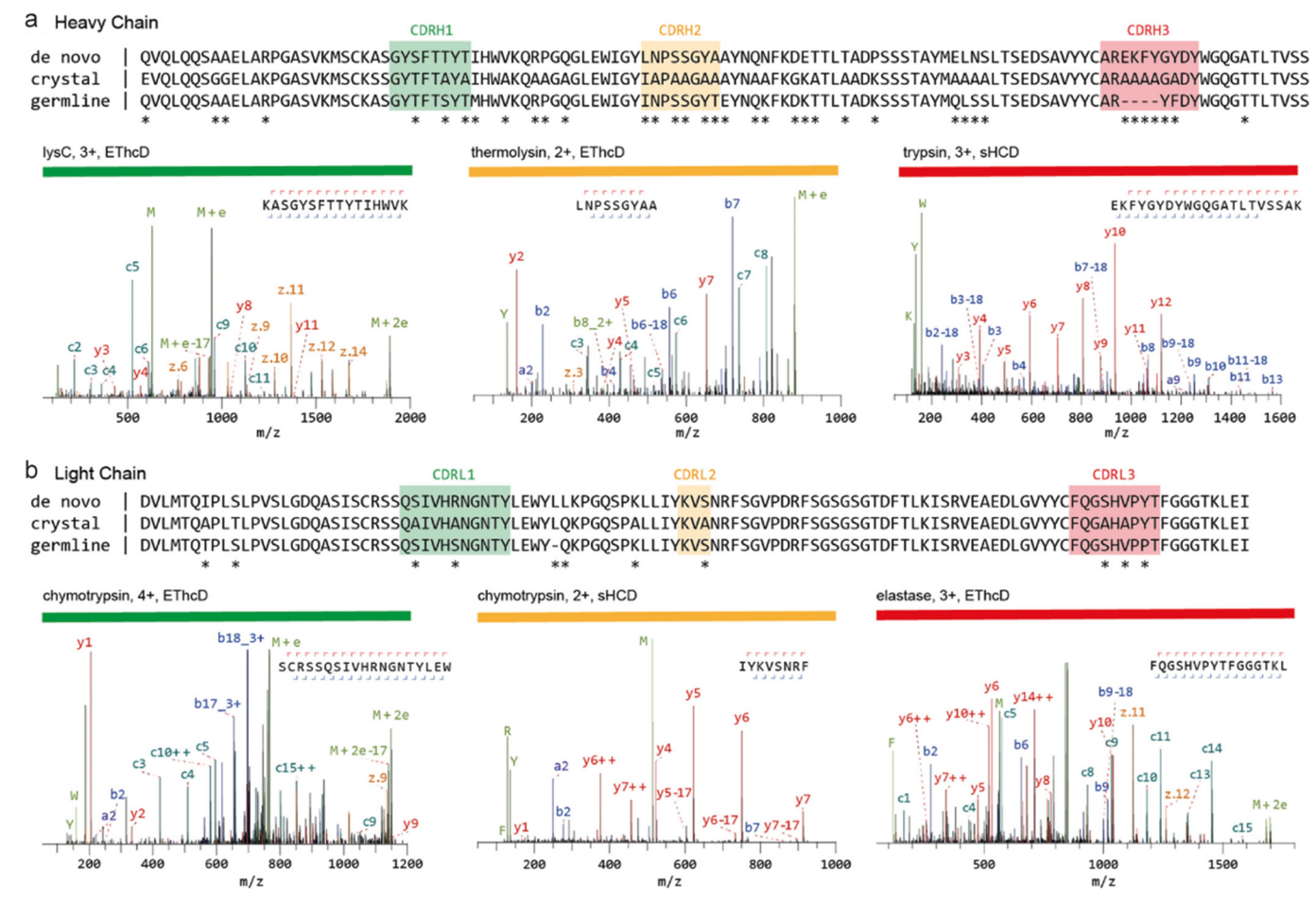
Dupré, M. et al. Anal Chem. 2021.
Figure 3. Sequencing of a Monoclonal Anti-FLAG M2 Antibody
Applications
1. Therapeutic Antibody Development: The antibody de novo sequencing service supports the design and optimization of therapeutic antibodies by providing precise sequence information for improving efficacy and safety.
2. Biosimilar and Biobetter Development: The antibody de novo sequencing service enables accurate characterization of reference antibodies, facilitating the development of biosimilars and next-generation biobetters.
3. Antibody Engineering: The antibody de novo sequencing service provides essential sequence data for engineering antibodies with enhanced binding affinity, stability, and reduced immunogenicity.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of Antibody De Novo Sequencing
5. Mass Spectrometry Image
6. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







