Premium Stem Cell-derived Exosome Production Service
- Regenerative medicine: Research used to promote the repair and regeneration of myocardial, nerve, skin and other tissues.
- Immunotherapy: Research used to modulate the immune system to treat autoimmune and inflammation-related diseases.
- Drug delivery: As a natural nanocarrier, it delivers small molecule drugs, RNA or proteins to improve therapeutic effects.
- Disease modeling: Study the role of exosomes in the occurrence and development of diseases and develop new treatment strategies.
Premium Stem Cell-derived Exosome Production Service is designed to provide high-purity, high-activity, and fully customizable stem cell-derived exosome preparation solutions to meet the diverse needs of basic research, drug development, biomaterials research, and regenerative medicine.
Stem cells have demonstrated great promise in tissue repair, immune regulation, and regenerative therapies. However, cell-based treatments face challenges such as tumorigenicity, immune rejection, and difficulties in storage and transportation. Recent studies have revealed that the therapeutic effects of stem cells are largely mediated through paracrine mechanisms—particularly via the secretion of exosomes.
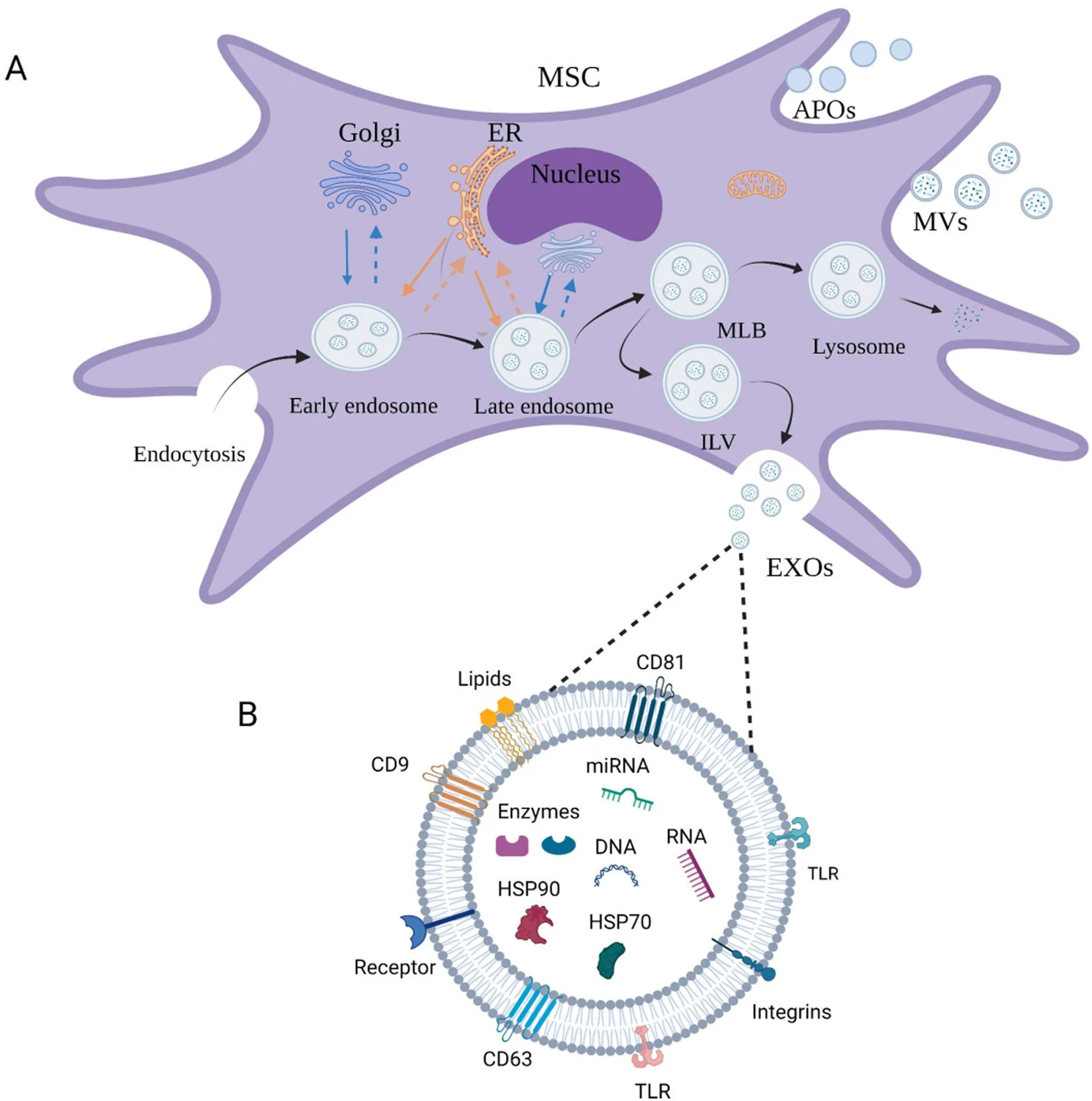
Kou M. et al. Cell Death Dis. 2022.
Stem Cell-Derived Exosomes are key mediators of stem cell bioactivity. Enriched with a variety of biologically active molecules such as proteins, lipids, mRNAs, and miRNAs, these exosomes exert functional roles in modulating inflammation, promoting tissue repair, inhibiting fibrosis, and regulating immune responses. Functionally, they serve as essential extracellular tools in tissue engineering and biotherapeutics. Compared to direct stem cell application, exosome-based therapies circumvent many ethical and safety concerns, offering greater safety, stability, and controllability. As a result, stem cell-derived exosomes have emerged as vital research targets in regenerative medicine, immunotherapy, and drug delivery.
Leveraging a robust exosome isolation and analysis platform, MtoZ Biolabs offers the Premium Stem Cell-derived Exosome Production Service including not only standardized exosome preparation, but also comprehensive quality control assessments covering morphology, particle size, signature markers, and functional validation. In addition, we offer personalized support such as large-scale production, targeted cargo loading, and functional evaluation tailored to meet clients’ specific experimental needs.
Service Workflow
Our Premium Stem Cell-derived Exosome Production Service includes the following key steps:
1. Stem Cell Culture
Appropriate stem cell types (e.g., mesenchymal stem cells, embryonic stem cells) are selected and expanded under optimized culture conditions.
2. Exosome Collection and Isolation
Stem cell culture supernatant is regularly collected, and high-purity exosomes are obtained using ultracentrifugation, density gradient centrifugation, size exclusion chromatography (SEC) and other methods.
3. Quality Control
Perform a comprehensive quality assessment of exosomes, including particle size analysis, purity testing, and activity verification, to ensure that they meet the needs of downstream research or applications.
Applications
MtoZ Biolabs’ Premium Stem Cell-derived Exosome Production Service supports a broad range of research areas:
Service Advantages
1. High-Quality Exosome Production: Utilizes standardized, traceable production workflows to ensure high purity and bioactivity of exosomes.
2. Customizable Project Design: Offers tailored production and analysis options aligned with the client’s experimental needs.
3. Strict quality control: Each batch of products undergoes strict quality testing to ensure that it meets the standards for scientific research or clinical application.
4. Professional technical support: We have an experienced scientific research team that provides comprehensive technical consultation and support to help customers solve problems encountered in exosome research and application.
FAQ
Q. How to Ensure the Quality and Purity of the Exosomes?
MtoZ Biolabs uses a variety of technologies to systematically verify the purity and integrity of exosomes, including particle size distribution detection (NTA), transmission electron microscopy (TEM) observation, exosome marker protein detection, protein contamination rate assessment, etc. Ensure that customers obtain high-purity, high-quality stem cell-derived exosomes, which are suitable for high-level scientific research or translational research.
Q. Have the stem cells been phenotypically verified and tested for contamination?
Yes, ensuring stem cell quality is a prerequisite for high-quality exosome production. The stem cells used by MtoZ Biolabs have undergone rigorous phenotypic verification, and flow cytometry is routinely used to detect the positive expression of CD73, CD90, and CD105, as well as the negative expression of hematopoietic markers such as CD45 and CD34. In addition, each batch of cells undergoes mycoplasma testing, endotoxin testing, and bacterial and fungal contamination screening to ensure the purity and biosafety of exosomes from the source.
Case Study
In this study, exosomes (hUC-MSC-Exos) were isolated from the conditioned media of human umbilical cord mesenchymal stem cells (hUC-MSCs) using ultracentrifugation. Characterization was performed via TEM, NTA, and Western blot analysis targeting exosomal markers CD63 and CD81. Functional assays revealed that hUC-MSC-Exos exert antifibrotic effects on human endometrial stromal cells (hESCs). The mechanism involved the delivery of miR-140-3p, which downregulated FOXP1 expression, thereby inhibiting Smad3 phosphorylation and reducing the expression of fibrosis-related genes. These findings suggest that hUC-MSC-Exos regulate the miR-140-3p/FOXP1/Smad axis to suppress fibrosis, offering a potential therapeutic strategy for endometrial fibrotic disorders.
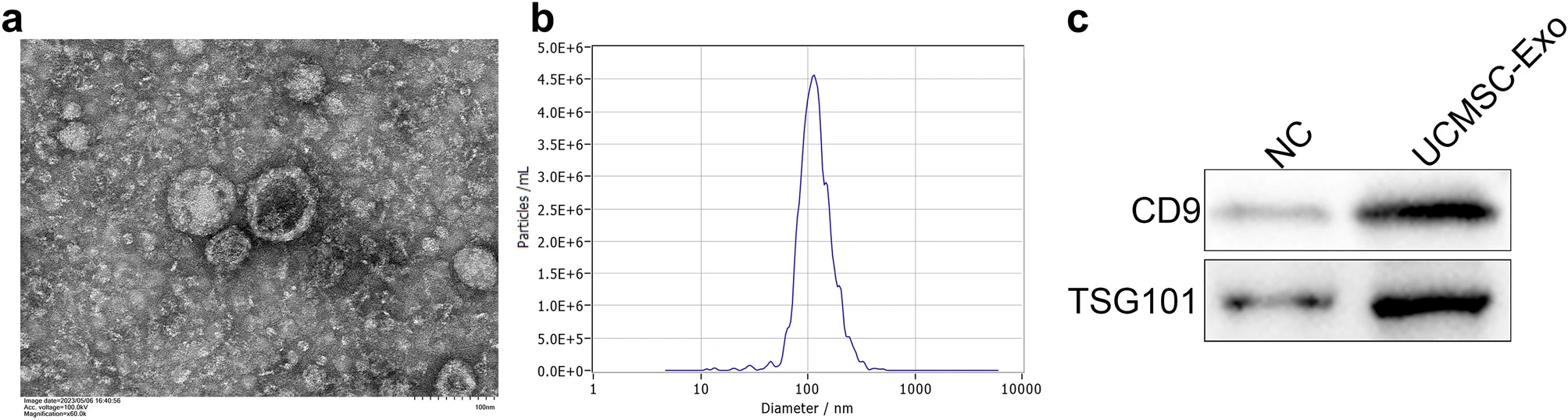
Song M. et al. Sci Rep. 2024.
How to order?







