Monoclonal Antibody Characterization Services
Monoclonal Antibody Characterization refers to the comprehensive analysis of the physicochemical properties, structural integrity, functional activity, and stability of monoclonal antibody (mAb). Its core principle involves utilizing high-resolution analytical techniques such as liquid chromatography-mass spectrometry (LC-MS), circular dichroism (CD), and differential scanning calorimetry (DSC) to thoroughly examine the amino acid sequence, post-translational modifications (PTMs), glycosylation patterns, and aggregation states of antibodies. Additionally, methods such as isoelectric focusing (IEF), surface plasmon resonance (SPR), and enzyme-linked immunosorbent assay (ELISA) are employed to assess the charge heterogeneity, affinity, and functional activity of antibodies, ensuring their quality and consistency.
Monoclonal antibody characterization services are widely applied in biopharmaceutical development, antibody drug research, diagnostic reagent development, and fundamental scientific research. For instance, in biopharmaceutical production, this service ensures batch-to-batch stability and consistency of antibodies while optimizing manufacturing processes. In preclinical studies, it is used to evaluate the immunogenicity, affinity, and specificity of antibodies, providing critical data for targeted therapies. Furthermore, monoclonal antibody characterization plays a crucial role in immunology research, protein engineering, and bioanalysis, offering robust technical support for the development, production, and quality control of antibody-related products.
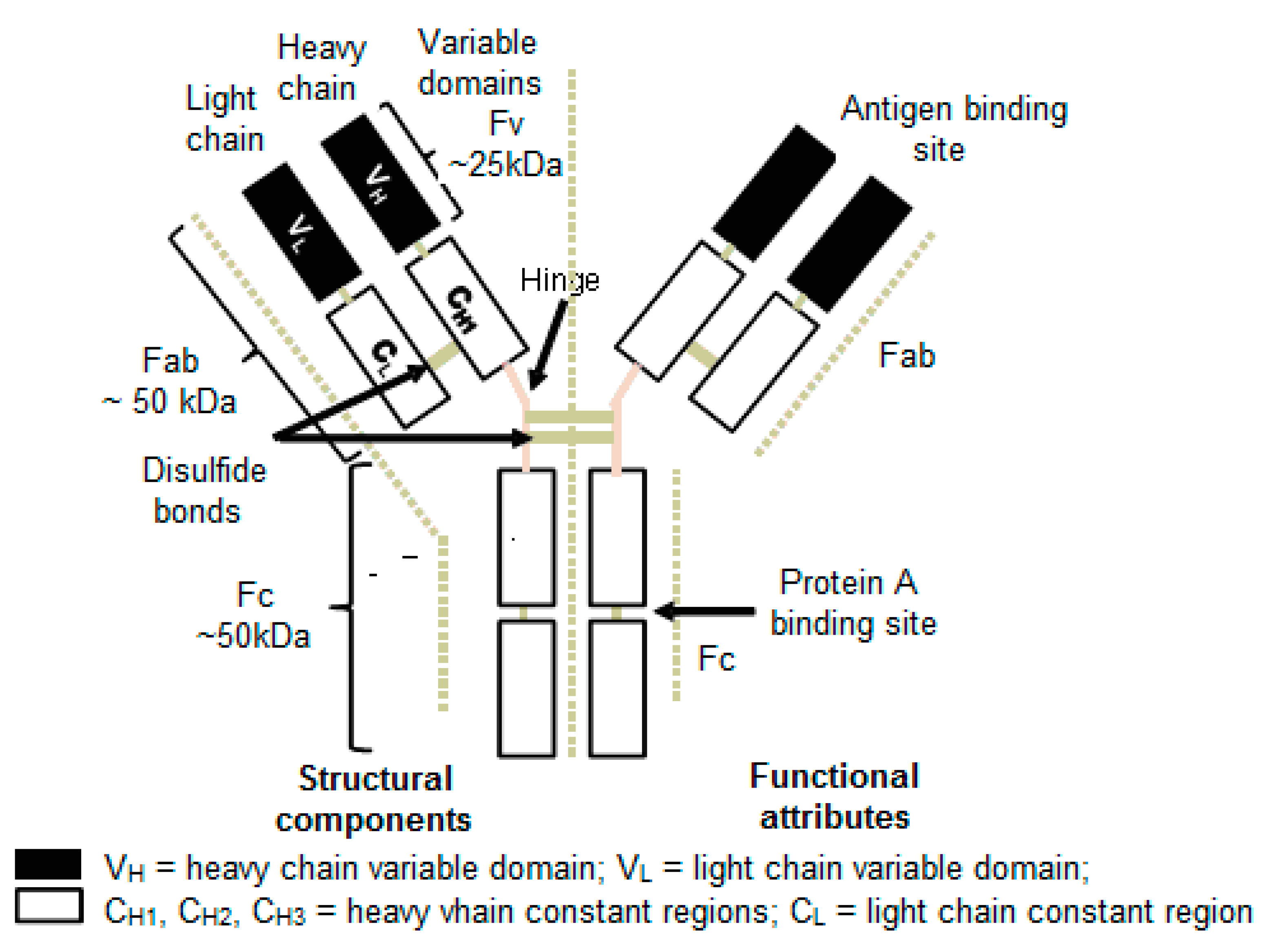
Alhazmi, H A. et al. Pharmaceuticals, 2023.
Figure 1. Structure of a Monoclonal Antibody (IgG).
Services at MtoZ Biolabs
MtoZ Biolabs offers professional monoclonal antibody characterization services using high-resolution mass spectrometry (LC-MS/MS) and advanced techniques to comprehensively characterize antibody amino acid sequences, post-translational modifications (such as glycosylation, oxidation, and deamidation), and higher-order structures (HOS). Our service includes purity analysis, heterogeneity evaluation, impurity detection, and antigen-binding validation, ensuring structural integrity and functional consistency of the antibodies. We provide high-resolution, high-sensitivity, and highly reproducible data support to assist clients in optimizing the development, production, and quality control of antibody drugs, facilitating advancements in biopharmaceuticals, preclinical research, and immunology studies.
Service Advantages
1. High-Resolution Analysis for Precise Characterization
MtoZ Biolabs leverages advanced analytical platforms to achieve comprehensive structural analysis of monoclonal antibodies, including sequence confirmation, post-translational modification (PTM) detection, and glycosylation analysis, ensuring data precision and reliability.
2. Multidimensional Detection for Comprehensive Coverage
Our services cover multiple key quality attributes (CQAs) of antibodies, providing a comprehensive antibody characterization solution to ensure the functional integrity and stability of antibody products.
3. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
4. High Sensitivity and Reproducibility
Utilizing advanced quantitative and qualitative analysis methods, we precisely detect low-abundance modifications and trace impurities, ensuring the consistency of antibody products while meeting the stringent requirements of biopharmaceutical development and quality control.
5. Customized Analytical Solutions
MtoZ Biolabs provides tailored analytical solutions for different antibody types (IgG, IgA, IgM, nanobodies, etc.) and diverse application needs (drug development, fundamental research, quality control), supporting antibody drug development and clinical translation.
Applications
1. Antibody Drug Development and Quality Control
Monoclonal antibodies are widely utilized in biopharmaceuticals, where their structure, modifications, and stability directly impact drug efficacy and safety. Monoclonal antibody characterization services enable sequence confirmation, post-translational modification (PTM) analysis, glycosylation profiling, aggregate assessment, and stability testing. These analyses ensure product consistency, meeting the stringent quality and regulatory requirements of biopharmaceutical development.
2. Biomarker Research and Diagnostic Reagent Development
In precision medicine and in vitro diagnostics (IVD), monoclonal antibodies are essential for biomarker detection and diagnostic reagent development. Through affinity analysis, binding specificity testing, and stability evaluation, antibody performance can be optimized to enhance detection accuracy, providing reliable support for clinical diagnostics.
3. Immunotherapy and Personalized Medicine
Monoclonal antibodies play a crucial role in precision treatment for cancer and immune disorders. By characterizing antigen-binding properties, Fc region modifications, and effector functions, antibody drug design can be optimized to enhance therapeutic efficacy and support the development of personalized immunotherapy.
4. Basic Research and Protein Interaction Analysis
In life sciences research, monoclonal antibodies are widely used for protein function studies and signaling pathway analysis. Monoclonal antibody characterization services assist researchers in analyzing antibody binding properties, protein interactions, and structural modifications, providing accurate data to support fundamental research.
Case Study
1. Comprehensive Molecular Characterization of a Cisplatin-Specific Monoclonal Antibody
This study aimed to comprehensively characterize a cisplatin-specific monoclonal antibody (mAb) to elucidate its binding mechanism and evaluate its potential applications in cisplatin detection and targeted therapy. The research focused on a monoclonal antibody specifically recognizing cisplatin. The study employed liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze the antibody’s sequence and post-translational modifications (PTMs), surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) to measure its binding affinity to cisplatin, X-ray crystallography to resolve the antibody-cisplatin complex structure, and cell-based assays to assess its targeting ability in cisplatin-treated cells. The results demonstrated that the antibody exhibited high-affinity binding to cisplatin-DNA adducts and effectively recognized intracellular cisplatin distribution. Crystal structure analysis identified the antibody’s cisplatin-binding site and key amino acid residues, revealing specific molecular interactions responsible for its high specificity. Further cellular experiments confirmed the mAb’s capability to detect intracellular cisplatin following treatment. The study concluded that this cisplatin-specific mAb holds potential applications in cisplatin drug research, clinical detection, and targeted delivery, providing new strategies for cisplatin drug optimization and personalized therapy.
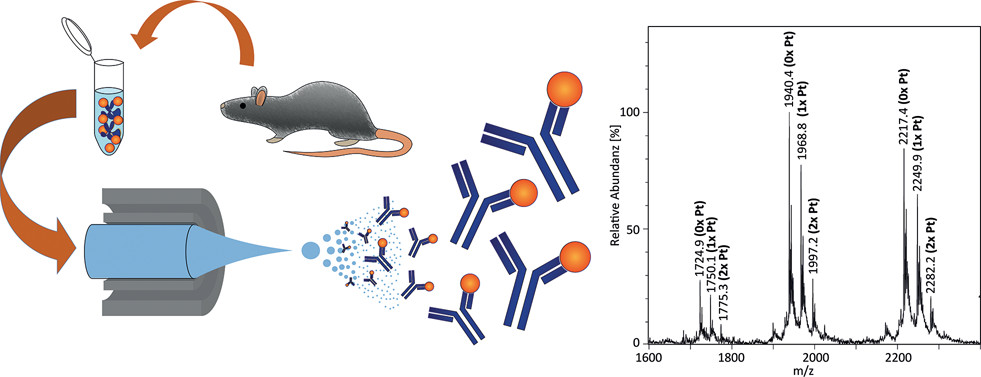
Ruhe, L. et al. Molecular Pharmaceutics, 2017.
Figure 2. The Workflow of the Study.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of Monoclonal Antibody Characterization
5. Mass Spectrometry Image
6. Raw Data
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
How to order?







