GPC/SEC-RI Based Polysaccharide Molecular Weight Identification Service
MtoZ Biolabs provides polysaccharide molecular weight characterization using advanced gel permeation / size exclusion chromatography coupled with refractive index detection (GPC/SEC RI). Size based fractionation combined with sensitive RI quantitation delivers detailed molecular weight distribution data, degree of polymerization readouts, and purity evaluation for polysaccharides and their derivatives. Our service supports quality control of natural and synthetic polysaccharides and is widely used in glycosylation studies, drug carrier development, and biomaterials research.
Technical Overview
Polysaccharides are ubiquitous biological macromolecules present in plants, animals, microorganisms, and a wide range of synthetic carbohydrate derivatives. They contribute to structure, signaling, immune modulation, and formulation performance, and they are increasingly engineered for pharmaceuticals, functional nutrition, and biomaterials. Accurate characterization of molecular weight and molecular weight distribution is essential for linking structure to biological function, optimizing synthetic or modification processes, and improving product performance.
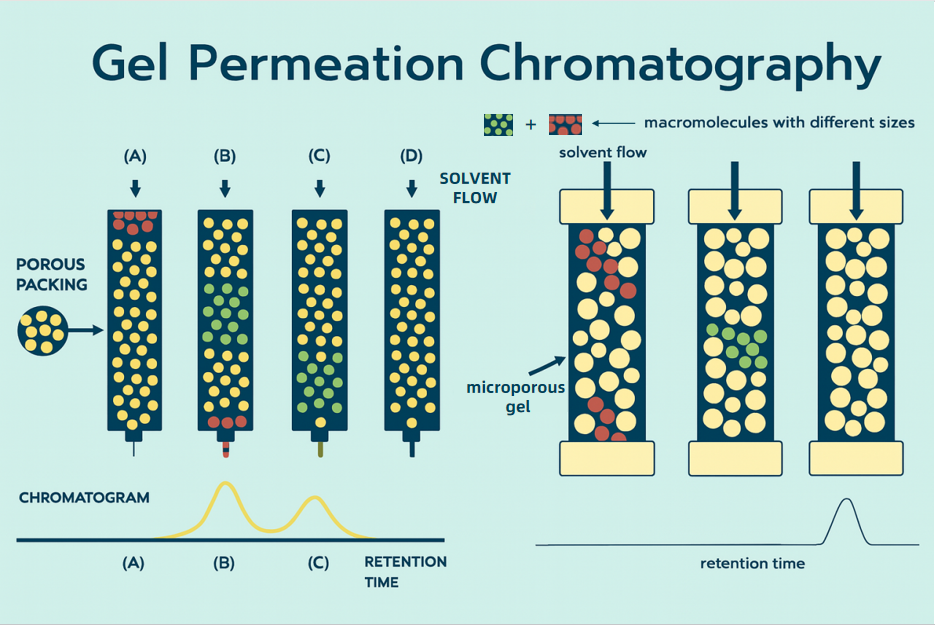
Size exclusion chromatography coupled with refractive index detection (SEC RI, also called GPC RI) is a well-established standard for polysaccharide molecular weight analysis. SEC separates components primarily by hydrodynamic size as analytes partition through a porous matrix. The refractive index detector measures solution refractive index changes that scale with analyte concentration, enabling reliable quantitation across broad mass and concentration ranges without a chromophore requirement. With proper calibration, SEC RI provides molecular weight distribution profiles, number and weight average molecular weights, degree of polymerization, polydispersity, and related physicochemical readouts. The approach is widely applied to natural and synthetic polysaccharides, glycosylated polymers, and glycoconjugate materials, supporting deeper insight in glycosylation research, drug carrier development, vaccine and adjuvant formulation, and biomaterial optimization.
Why Choose MtoZ Biolabs
• Highresolution size profiling. Separation is driven by hydrodynamic volume, so samples do not need intrinsic absorbance. Heterogeneous materials—including natural, synthetic, branched, or chemically modified polysaccharides—can be profiled with confidence.
• Labelfree RI quantitation. Refractive index detection reads native refractive changes without dyes, fluor tags, or chemical derivatization, helping preserve labile linkages and native structure.
• Wide molecular weight coverage. Multipoint calibration supports measurements from lowkilodalton oligos through highmegadalton carbohydrate polymers, covering development needs from early synthesis to final product QC.
• Flexible, sampleaware methods. Workflows are adjusted for solubility, viscosity, aggregation state, and degree of polymerization. Multiple column sets and buffer systems accommodate natural polysaccharides, hydrogeltype carbohydrate polymers, and glycoprotein side chains.
• Complete, traceable data package. Deliverables include full chromatograms, molecular weight distribution plots, and key statistics (Mn, Mw, Mz, dispersity, degree of polymerization, and more) with method parameters documented for review and repeatability.
Types of Polysaccharides Analyzed by MtoZ Biolabs
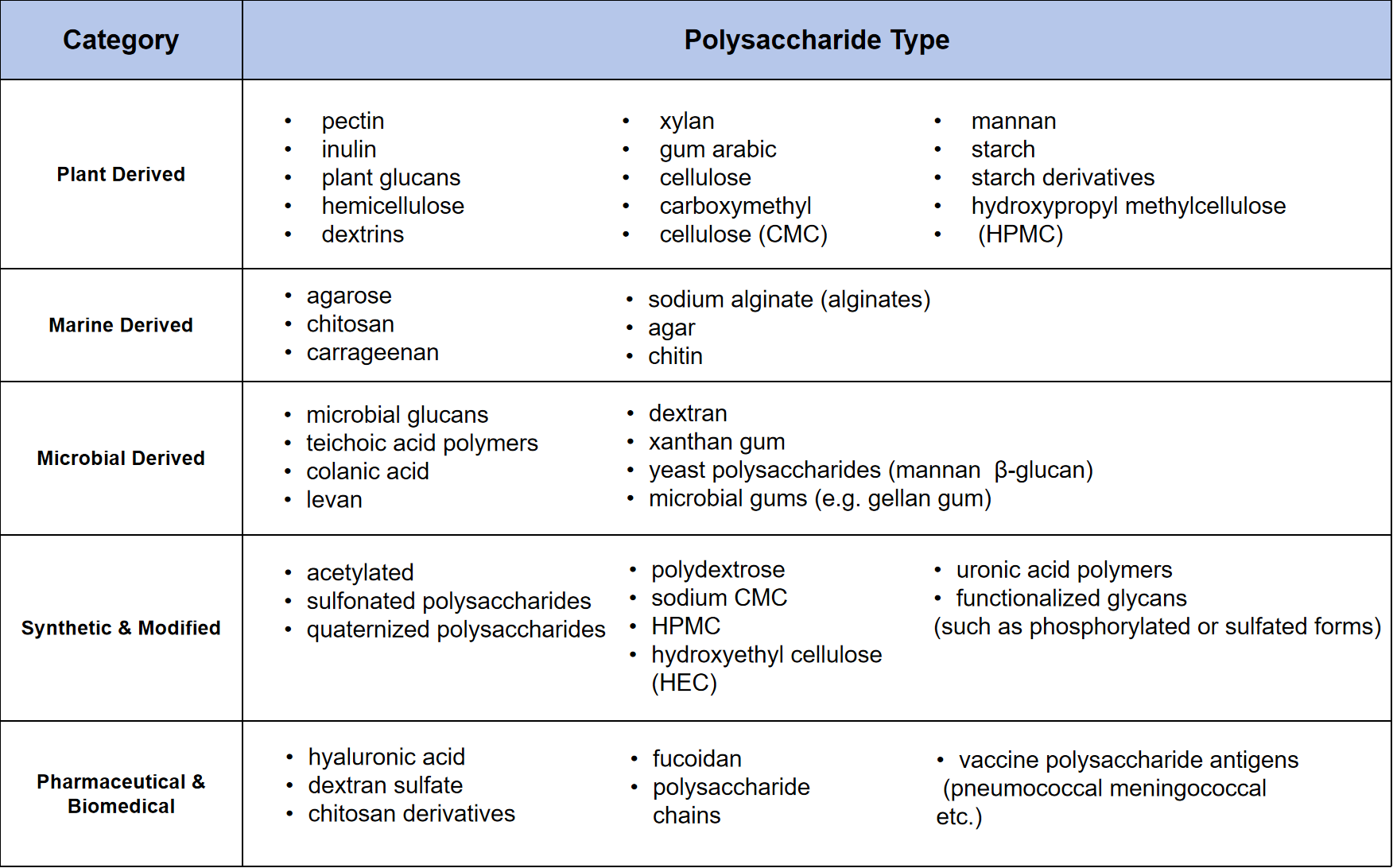
If your sample type does not appear above, please contact the MtoZ Biolabs technical support team to confirm analytical feasibility.
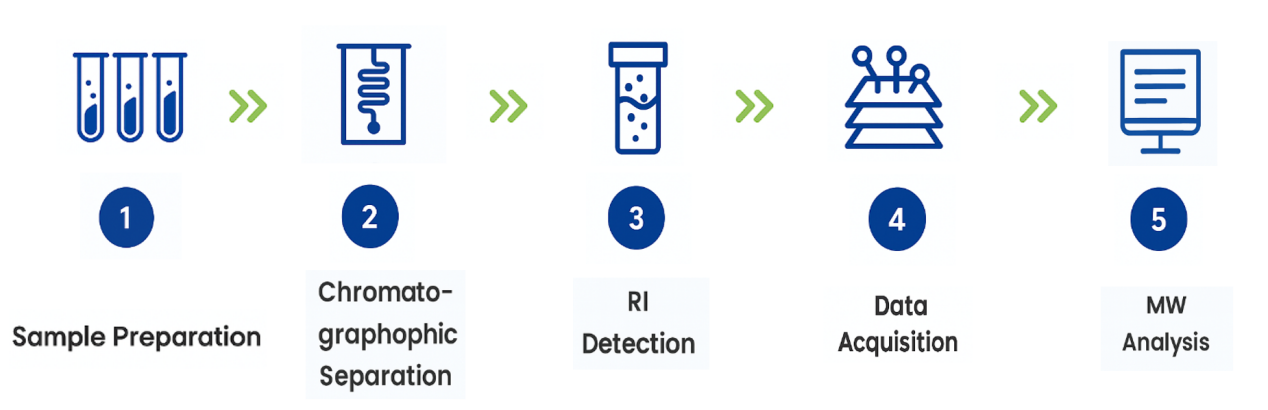
Analysis Workflow
Applications
1. Synthetic Polysaccharide Characterization:
Analyze molecular weight and degree of polymerization of synthetic polysaccharides to ensure consistent quality throughout the synthesis process.
2. Natural Polysaccharide Analysis
Evaluate molecular weight distribution of natural polysaccharides—such as those derived from plants and algae—to support raw material quality control.
3. Glycosylation Research
Characterize structural features of glycosylated polysaccharides to provide critical data for glycosylation studies.
4. Polysaccharide Drug Analysis
Assess polysaccharidebased therapeutics and biomaterials—including hyaluronic acid, chitosan, and glucan—for formulation development and product evaluation.
Sample Submission Suggestions
Sample quality:
Dry powder or lyophilized samples: 5–10 mg; liquid samples: ≥ 500 µL.
Purity:
Recommended purity of at least 90 percent. Samples should be free of high concentrations of salts, surfactants, organic buffers, or other additives.
If you have unusual or poorly soluble polysaccharides, please contact our technical team prior to submission for customized pretreatment recommendations and detailed submission guidelines.
Deliverables
• Molecular weight distribution plot: Visual profile showing the full molecular weight spectrum of the polysaccharide sample.
• Key statistical parameters: Report of Mn, Mw, Mz, dispersity (Mw/Mn), and degree of polymerization (DP) with calculation notes.
• Raw chromatogram and data files: Complete chromatographic traces and underlying raw data for independent review or reprocessing.
• Method conditions summary: Column set, mobile phase composition, flow rate, detector settings, calibration standards, and other relevant analytical parameters.
How to order?







