Overview
The mutation of the end of the antibody drug is divided into the N terminal and the C terminal variations. The common variation at the N end is usually due to incomplete processing of the N- terminal signal sequence, resulting in the N- terminal methionine residue being not removed or the N- terminal Glu and Gln residues partially or completely forming pyroglutamic acid. The common mutations at the C end are sequence truncation, such as the deletion of the C terminal Lys residue of the heavy chain in the monoclonal antibody, which is also known as K deletion. These changes in the protein terminals may occur in the fermentation conditions, or caused by condition change in the protein purification buffer. For example, it has been reported that the monoclonal antibody derived from B cell hybridoma and CHO cell transfected tumor have a very significant variation in the proportion of C- lysine deletion. Another case is that when a monoclonal antibody is being modified, the ratio of lysine to the C-terminal end also changed significantly. These two common cases reveal the importance of analyzing the c-terminal variation. In the ICH Q6B guideline, C terminal K deletion detection is mandatory. The methods of IEF, cIEF, ion exchange chromatography and LC-MS are common in analyzing the variation of lysine at the C-terminal end. MtoZ Biolabs uses LC-MS for antibody C-terminal variation analysis, including detection of the proportion of K deletion in the C-terminal end of antibody and other types of truncation on antibody C-terminal.
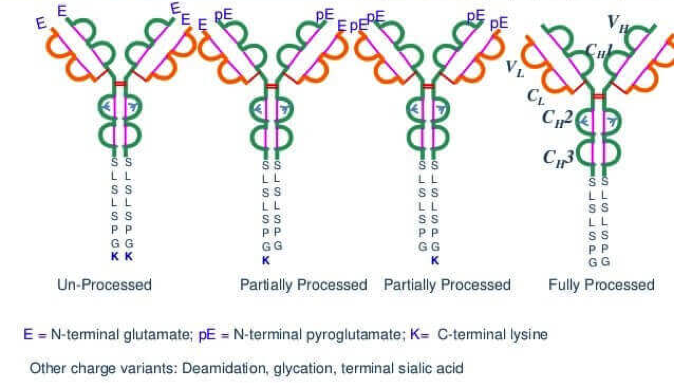 Antibody C-terminal Variations
Antibody C-terminal Variations
Reports
• Experiment procedures
• Parameters of liquid chromatography and mass spectrometer
• MS raw data files
• Antibody c-terminal variation results
• Bioinformatics analysis
 Related Services
Related Services

 |
Address: 155 Federal Street, Suite 700, Boston, MA 02110, USA |
 |
Email: info@MtoZ-Biolabs.com |
 |
Fax: 1-617-616-8054 |